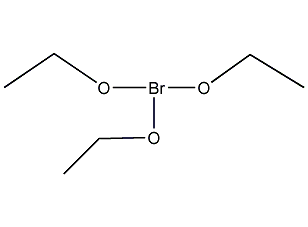
Structural formula
| Business number | 03Y7 |
|---|---|
| Molecular formula | C6H15BO3 |
| Molecular weight | 145.99 |
| label |
Triethoxyborane, Boric acid triethyl ester, Boron ethoxide, Triethoxyborane, plasticizer, welding flux |
Numbering system
CAS number:150-46-9
MDL number:MFCD00009073
EINECS number:205-760-9
RTECS number:ED5075000
BRN number:1699052
PubChem number:24900331
Physical property data
1. Properties: Colorless and transparent liquid, easy to absorb moisture.
2. Density (g/mL, 20/4ºC): 0.8546
3. Melting point (ºC): -84.5
4. Flash point (ºC ): 11
5. Boiling point (ºC, 101.3KPa): 120
6. Refractive index (20ºC): 1.3749
7. Solubility: energy Miscible with ethanol and ether, decomposes when exposed to water.
Toxicological data
1. Acute toxicity: Mouse oral LD50: 2100 mg/kg
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 38.51
2. Molar volume (cm3/mol): 168.6
3. Isotonic specific volume (90.2K): 366.8
4. Surface tension (dyne/cm): 22.3
5. Polarizability (10-24cm3): 15.26
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 55.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure. Incompatible materials: strong oxidants, strong acids. It is flammable and can cause combustion when exposed to open flames or high heat.
Storage method
Should be stored sealed in a cool, dry place. Store at 0-6℃.
Synthesis method
1. It can be prepared by reacting ethanol and boron anhydride at 80℃. Since this product is hydrolyzed in water, a water-absorbing agent is added during the reaction to keep the system dry. After the reaction is completed, it is distilled and refined.
2. Preparation method:
In a reaction bottle equipped with a stirrer, reflux condenser, and thermometer, add absolute ethanol 460mL, add 75g (1.08mol) of boron oxide powder (2) in batches at a rate such that the temperature of the reaction solution rises without refluxing, and the addition is completed in about 10 minutes. Cool and filter out the solid boric acid. The filtrate was fractionated, and about 250 mL of azeotrope of triethyl borate and ethanol was evaporated. Wash three times with cold concentrated sulfuric acid, and then fractionate. Collect the fractions at 118-120°C to obtain 50 g of compound (1), with a yield of 30%. [1]
Purpose
It is used in the manufacture of semiconductor components and the synthesis of other organic borides. It is also used as high-purity raw materials, plasticizers and welding fluxes.

 微信扫一扫打赏
微信扫一扫打赏

