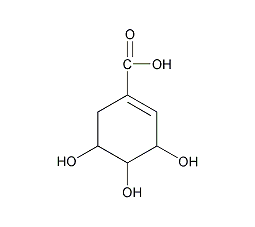
Structural formula
| Business number | 03S1 |
|---|---|
| Molecular formula | C7H10O5 |
| Molecular weight | 174.15 |
| label |
(3R,4S,5R)-3,4,5-trihydroxy-1-cyclohexenecarboxylic acid, (3R,4S,5R)-3,4,5-Trihydroxy-1-cyclohexenecarboxylic acid, aromatic compounds, acidic solvent |
Numbering system
CAS number:138-59-0
MDL number:MFCD00066278
EINECS number:205-334-2
RTECS number:GW4600000
BRN number:4782717
PubChem number:24899647
Physical property data
1. Properties: Colorless or white needle-like crystals.
2. Solubility: Solubility in water at 20ºC is 18%, slightly soluble in ethanol and ether, almost insoluble in chloroform and benzene.
3. Boiling point (ºC): 400.5
4. Melting point (ºC): 185~187
5. Flash point (ºC): 210.1
p>
6. Relative density (d204) : 1.725
Toxicological data
Acute toxicity data:
Mouse intraperitoneal LD: 1 mg/kg
Tumorigenicity data:
Mouse oral TDLo: 4000mg/kg
Mouse abdominal cavity TDLo: 400mg/kg
Mutagenicity data:
Mouse abdominal cavity dominant lethality test: 1000mg/kg
Oral dominant lethality test in mice: 3200mg/kg
Morphological transformation test in hamster kidney: 250mg/L
Ecological data
None
Molecular structure data
Molecular property data:
1. Molar refractive index: 38.14
2. Molar volume (cm3/mol): 100.9
3. Isotonic specific volume (90.2K): 331.7
4. Surface tension (3.0 dyne/cm): 116.6
5. Polarizability (0.5 10-24cm3): 15.12
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -1.7
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 5
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 98
p>
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 222
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 3
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Hygroscopic. Can sublimate
2. Exists in tobacco leaves.
Storage method
It should be sealed with argon and stored in a cool, dry place.
Synthesis method
Shikimic acid is derived fromA monomeric compound extracted from the medicinal star anise.
Purpose
Shikimic acid affects the metabolism of arachidonic acid, inhibits platelet aggregation, inhibits arterial and venous thrombosis and cerebral thrombosis, and has anti-inflammatory and analgesic effects. It can also be used as an antiviral and anticancer drug intermediate. This product is mostly used as a pharmaceutical intermediate. It has certain irritation and should not be used directly. Organic Synthesis

 微信扫一扫打赏
微信扫一扫打赏

