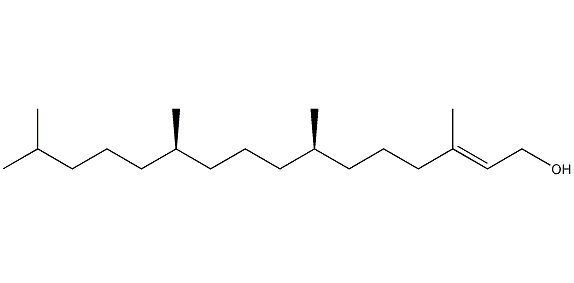
Structural formula
| Business number | 03YH |
|---|---|
| Molecular formula | C20H40O |
| Molecular weight | 296.53 |
| label |
(E)-3,7,11,15-Tetramethyl-2-hexadecen-1-ol, phytol, Phytol, (E)-3,7,11,15-Tetramethyl-2-hexadecenol, Naturalphytol, 3,7,11,15-Tetramethyl-2-hexadecen-1-ol, oxidizing agent, alcohol solvent |
Numbering system
CAS number:150-86-7
MDL number:None
EINECS number:205-776-6
RTECS number:TJ3490000
BRN number:1726098
PubChem ID:None
Physical property data
1. Physical property data:
1. Character: colorless or light yellow oily viscous liquid with weak floral and balsamic aroma.
2. Density (g/mL, 25/4℃): 0.8497
3. Refractive index (nD20): 1.463
4. Flash point ( ℃): 157.5
5. Boiling point (ºC, 1.33kpa): 335.5, 202~204℃ (1333pa)
6. Boiling point (ºC, 3.99pa): 145
7. Solubility: Miscible with organic solvents, insoluble in water.
8. Melting point (ºC): 140
Toxicological data
II. Toxicology data:
1. Acute toxicity: Rat oral LD50: >5 gm/kg;
Rabbit dermal LD50: >5 gm/kg .
Ecological data
3. Ecological data:
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 96.01
2. Molar volume (cm3/mol): 350.7
3. Isotonic specific volume (90.2K): 820.1
4. Surface tension (dyne/cm): 29.8
5. Polarizability (10-24cm3): 38.06
Compute chemical data
IV. Calculated chemical data:
1. Hydrophobic parameter calculation reference value (XlogP): 8.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 13
5. Topological molecular polar surface area (TPSA): 20.2
6. Number of heavy atoms: 21
7. Surface charge: 0
8. Complexity: 255
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters: 2
11. Uncertain number of atomic stereocenters: 0
12. Determine the number of chemical bond stereocenters: 1
13. The number of uncertain chemical bond stereocenters: 0
14. The number of covalent bonding units: 1
p>
Properties and stability
1. Stable under normal temperature and pressure.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
3. Naturally found in jasmine essential oil and tea leaves.
Storage method
Store sealed in a dry and cool place.
Synthesis method
1. Extracted from silkworm excrement.
2. Using chlorophyll as raw material, it is produced by vacuum distillation of its alkaline oxidative decomposition products.
3. Tobacco: FC, 9, 18, 40; FC, BU, OR, 14; Synthesis: Can be obtained from the intermediates for the synthesis of vitamin E.
Purpose
1. Biochemical research and use in the synthesis of vitamin E and vitamin K1.
2. Intermediates for the synthesis of vitamin K1 and vitamin E.
3. Used as the basic raw material for the production of vitamin K1, vitamin E, etc.
4. Used as the basic raw material for the production of vitamin K1, vitamin E, etc.
5.Used in lipstick, lipstick, foundation stick, hair wax, eyebrow pencil, foundation, eye makeup, rouge and other products, it feels good to use. Makeup is durable and reduces skin irritation. In addition, it can also be used as a W/O emulsifier to make the cream stable and less likely to deteriorate. It also has a good antioxidant effect and can also be used as a food emulsifier, antioxidant, and nutritional additive.
6. It can be used as a fixative in daily fragrance formulas.

 微信扫一扫打赏
微信扫一扫打赏

