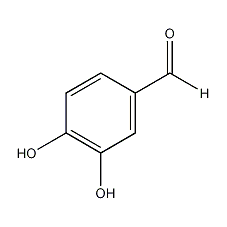
Structural formula
| Business number | 03SH |
|---|---|
| Molecular formula | C7H6O3 |
| Molecular weight | 138.12 |
| label |
1,2-Dihydroxy-4-formylbenzene, proto-tetraaldehyde, Protocatechualdehyde, Protocatechuicaldehyde, 1,2-Dihydroxy-4-formybenzene, aromatic compounds |
Numbering system
CAS number:139-85-5
MDL number:MFCD00003370
EINECS number:205-377-7
RTECS number:UL0380000
BRN number:774381
PubChem number:24893348
Physical property data
1. Melting point (ºC): 150~153
2. Relative density (d204) : 1.409
Toxicological data
Acute toxicity data:
Mouse abdominal LD50: 205mg/kg
Mouse intravenous LD50: 56mg/kg
Ecological data
None yet
Molecular structure data
Molecular property data:
1. Molar refractive index: 36.76
2. Molar volume (cm3/mol): 97.9
3. Isotonic specific volume (90.2K): 282.3
4. Surface tension (3.0 dyne/cm): 69.0
5. Polarizability (0.5 10-24cm3): 14.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 12
6. Topological molecule polar surface area 57.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 124
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is found in the leaves of Pteridium spp., a plant in the family Lepidaceae, in the leaves of the holly family, Salvia miltiorrhiza, a plant in the labiatae family, and other plants.
2. Exist in flue-cured tobacco leaves, oriental tobacco leaves, and mainstream smoke.
Storage method
Store in a sealed, dry, and light-filled state with nitrogen.
Synthesis method
1. Prepared from 3,4-methylenedioxybenzaldehyde through the following steps. Add the newly treated phosphorus pentachloride to 3,4-methylenedioxybenzaldehyde in batches. The reaction is very violent at the beginning and needs to be cooled with ice water. At the same time, moisture intrusion should be prevented. After adding approximately half of the phosphorus pentachloride, the strain slows down and cooling is no longer possible. The dosage of phosphorus pentachloride is 3 times (mole) of 3,4-methylenedioxybenzaldehyde. The resulting reaction solution was slowly heated for 1 hour to release hydrogen chloride, and then the volatilization solution was extracted under reduced pressure.�. Then, the reactants are poured into cold water, and the precipitated emulsified oil layer is left to solidify. Boil slowly for 3 hours, add activated carbon to filter, concentrate the filtrate under reduced pressure, cool to 0°C, and precipitate crystals. After filtering, washing with water and recrystallizing with water, the finished product is obtained.
2. Tobacco: OR, 26; FC, 54.
Purpose
Used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

