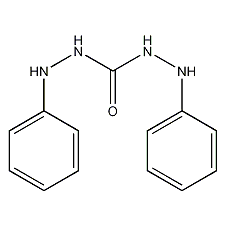
Structural formula
| Business number | 03ST |
|---|---|
| Molecular formula | C13H14N4O |
| Molecular weight | 242.28 |
| label |
1,5-Diphenylcarbohydrazide, sym.-Diphenylcarbazide, Diphenyl semicarbazide; diphenylcarbazide; 1,5-diphenylcarbonyl hydrazine;, aromatic compounds |
Numbering system
CAS number:140-22-7
MDL number:MFCD00003013
EINECS number:205-403-7
RTECS number:FF2750000
BRN number:752039
PubChem number:24894160
Physical property data
1. Properties: white crystalline powder.
2. Melting point ℃: 170-175
3. Solubility: slightly soluble in water, soluble in ethanol, acetone, insoluble in ether.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
Molecular property data:
1. Molar refractive index: 72.26
2. Molar volume (cm3/mol): 187.4
3. Isotonic specific volume (90.2K): 521.3
4. Surface tension (3.0 dyne/cm): 59.7
5. Polarizability (0.5 10-24cm3): 28.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.1
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 2
6. Topological molecule polar surface area 65.2
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 223
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
With Ag+ , Ni2+ , Pd2+ , Cu2+ , Fe Metal ions such as 3+, Hg+, Hg2+ have color reactions.
Storage method
Stored in a cool, dry place and sealed.
Synthesis method
1. Obtained from the reaction of phenylhydrazine and urea. Add phenylhydrazine and urea to xylene, reflux for 32 hours, leave overnight, and filter out the crude product. Then dissolve it with a mixed acid solvent of ethanol and a little acetic acid, quickly cool and crystallize, filter it, wash it with ethanol again, and filter it dry to get the finished product
2.Reflux and heat phenylhydrazine and a slight excess of urea to 155~160℃ until the reactants are completely melted and the bubbles disappear:
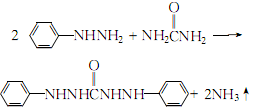
Keep warm for 2 to 3 hours, and the reaction will end. After cooling slightly, add 95% ethanol to precipitate part of the diphenylcarbamoyl dihydrazide, then reheat and reflux until the crystals are completely dissolved, filter while hot, cool the filtrate with ice salt to crystallize, filter with suction and wash with a small amount of ethanol until it is qualified. . Filter and dry at 100°C to obtain diphenylcarbonyl dihydrazide.
Purpose
1. Used as a redox indicator for the colorimetric determination of chromium, mercury, lead, dichromate determination, adsorption indicator for the determination of chloride and cyanide by mercury measurement, and for the determination of cadmium, copper, iron, molybdenum and Vanadium etc.
2It is also used as an indicator for redox, adsorption and coordination titration.

 微信扫一扫打赏
微信扫一扫打赏

