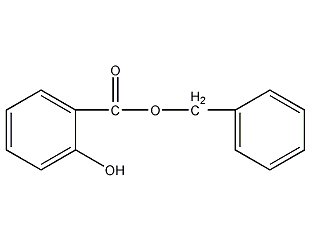
Structural formula
| Business number | 039Y |
|---|---|
| Molecular formula | C14H12O3 |
| Molecular weight | 228.24 |
| label |
Benzyl salicylate, Benzyl 2-Hydroxybenzoate, Benzyl ortho-hydroxybenzoate, Benzyl salicylate, Salicylic acid benzyl ester, Benzyl 2-hydroxybenzoate, spices, Nitromusk solution, fixative, Bactericidal preservatives |
Numbering system
CAS number:118-58-1
MDL number:MFCD00020034
EINECS number:204-262-9
RTECS number:VO1750000
BRN number:2115365
PubChem number:24888191
Physical property data
1. Properties: white crystalline powder, colorless liquid when the temperature is high, with a slightly sweet aroma.
2. Relative density: 1.176~1.179 (25℃)
3. Melting point (ºC): 24~26
4. Boiling point (ºC, 101.3 kPa): 320
5. Boiling point (ºC, 3.16kPa): 208
6. Boiling point (ºC, 1.33kPa): 186~188
7 . Refractive index (20ºC): 1.580~1.581
8. Flash point (ºC): 167
9. Solubility: Insoluble in water, soluble in ethanol.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2227mg/kg
2. Toxic, irritating to the gastrointestinal tract.
Ecological data
Slightly harmful to water.
Molecular structure data
1. Molar refractive index: 64.39
2. Molar volume (cm3/mol): 186.4
3. Isotonic specific volume (90.2K ): 499.2
4. Surface tension (dyne/cm): 51.4
5. Polarizability (10-24cm3): 25.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 4
6. Topological molecule polar surface area 46.5
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 246
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable at room temperature and pressure, avoid contact with oxides.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. should be kept away from oxidizer, do not store together.
2. Equip with corresponding varieties and quantities of fire-fighting equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Obtained from the reaction of salicylic acid and benzyl alcohol.
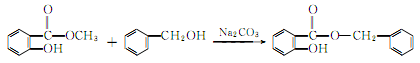
2. It can be obtained by the reaction of sodium salicylate and benzyl chloride. Add a small amount of diethylamine to the mixture of sodium salicylate and a slight excess of benzyl chloride, heat to 130-140°C and react for 17 hours. After cooling, wash the reactant with water. Excess benzyl chloride is removed by steam distillation, and the remaining The oil is vacuum distilled and the 170-175°C (0.93kPa) fraction is collected. Yield 85%.
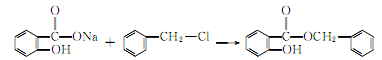
2. From salicylic acid and Derived from esterification of phenylethyl alcohol.
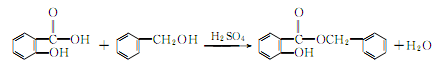
Purpose
1. Mainly used for preparing apricot, peach, banana, raspberry and other flavors. It can also be used as a bactericidal preservative. A good solvent for the synthesis of nitromusk. It is a good fixative for floral scents such as clove, carnation, lily of the valley, and jasmine. It is also used in small amounts in food flavors to prepare raisin and red currant flavors. Product quality: content ≥98%.
2.Used as ultraviolet absorber, spice, fixative and bactericidal preservative.
3. Used for the preparation of spices, suitable for the preparation of solvents for artificial nitro musk and fixatives for floral fragrances.

 微信扫一扫打赏
微信扫一扫打赏

