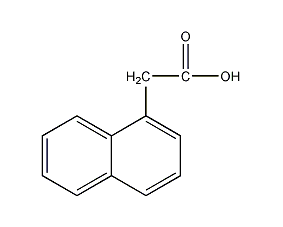
Structural formula
| Business number | 01X5 |
|---|---|
| Molecular formula | C12H10O2 |
| Molecular weight | 186 |
| label |
1-Naphthylacetic acid, α-naphthylacetic acid, α-Naphthaleneacetic acid, 1-Naphthylacetic acid, NAA, Plant growth regulators; aromatic carboxylic acids and their derivatives |
Numbering system
CAS number:86-87-3
MDL number:MFCD00004046
EINECS number:201-705-8
RTECS number:QJ0875000
BRN number:1308415
PubChem number:24897435
Physical property data
1. Properties: White needle-like crystals or crystalline powder, odorless.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 134.5~135.5
5. Crystal phase standard combustion heat (enthalpy) (kJ·mol-1): -5792.1
6. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -359.2
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC) : Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13 . Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion Lower limit (%, V/V): Undetermined
19. Solubility: Soluble in hot water, ether, acetone, chloroform benzene, acetic acid and Alkaline solution, slightly soluble in cold water and ethanol.
Toxicological data
1. Acute toxicity:
Rat caliber LD50: 1mg/kg; rat inhalation LC50: >207 gm/m3
Rat abdominal LD50: 100mg/kg;
Mouse caliber LD50: 743mg/kg; Mouse subcutaneous LD50: 733mg/kg;
Mouse abdominal LD50: 609mg/kg;
Rabbit skin LD50: >5mg/kg;
2. Teratogenicity
Yeast: 500mg/L:
Human leukocytes: 100nmol/L
3. Other multiple dose toxicity data
Rat caliber DL0: 27mg/kg/90D-C;
Rat caliber DL0: 30mg/kg/30D-I;
4. Neurotoxicity
Rabbit eye test: 100 mgREACTION
Ecological data
General remarks
Water hazard class 1 (German Regulation) (self-assessment via list) This substance is hazardous to waterSlightly harmful.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 55.20
2. Molar volume (cm3/mol): 150.9
3. Isotonic specific volume (90.2K): 409.4
4. Surface tension (dyne/cm): 54.0
5. Polarizability (10-24cm3): 21.88
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 212
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product is moderately toxic, irritates the skin and mucous membranes, and has a paralyzing effect on the central nervous system. Protection should be paid attention to during the production process, especially in the drying section, which should be well ventilated to prevent dust from flying. Operators should wear labor protection equipment.
Storage method
This product should be dried and sealed, and stored in a dark place to prevent deterioration.
Industrial raw powder is packed in plastic bags and sacks. The fine products are packed in sacks or wooden barrels lined with plastic bags. Store in a cool and dry place. Fireproof and moisture-proof. Store and transport according to regulations on toxic substances.
Synthesis method
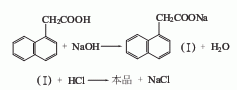
1. Generally obtained by the reaction between naphthalene and monochloroacetic acid. Naphthalene and monochloroacetic acid (molar ratio 3:1) react in the presence of aluminum powder, potassium bromide and other catalysts at 185~210℃ for several hours to form The hydrogen chloride gas is absorbed with water, leached with 5% to 6% sodium hydroxide solution, cooled and filtered, and excess naphthalene is recovered by steam distillation. The filtrate is neutralized (PH7) and acidified (PH56, PH3) with 31% to 31% hydrochloric acid. Filter to obtain a crude product, and recrystallize to obtain a refined product. It can also be obtained by chloromethylation, cyanation and hydrolysis of naphthalene.
2.Naphthalene is obtained by reacting with acetic anhydride in the presence of potassium permanganate.
3.Naphthalene and monochloroacetic acid react at 185~210℃ in the presence of aluminum powder, potassium bromide and other catalysts, After neutralization, acidification, and filtration, the crude product is obtained, and then refined naphthalene acetic acid is obtained through recrystallization. It can also be obtained by chloromethylation, cyanation and hydrolysis of naphthalene.
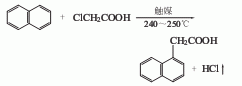
Purpose
1. Plant growth regulator. Used in organic synthesis and used as plant growth stimulant. It is used as a plant growth stimulant in agriculture. It has the characteristics and physiological functions of the endogenous auxin indole acetic acid, which promotes crop metabolism and photosynthesis, such as promoting cell division and expansion, inducing the formation of unstable roots, increasing fruit set, and preventing Fruit drop, changing the ratio of male and female flowers, etc. It enters the plant through the tender epidermis of leaves, branches, seeds, etc., and is guided to the active part along with the flow of nutrients. At higher concentrations, it has the effect of inhibiting growth. Soaking rice seedlings and wheat seeds can increase yields. It can also prevent the shedding of fruit trees and cotton, promote the rooting and flowering of various plant cuttings, increase the germination rate, and make crops mature early and be productive. Prevent flower and fruit drop and the formation of seedless fruits.
2.Plant growth stimulating hormone. Used in enzyme chemistry for the determination of α-esterase. Also used in organic synthesis.
3.Broad-spectrum plant growth regulator. It can promote cell division and expansion, induce the formation of adventitious roots, increase fruit setting, prevent fruit drop, change the ratio of female and male flowers, etc. Naphthalene acetic acid can enter the plant body through the tender epidermis of leaves, branches, and seeds, and is transported to the site of action along with nutrient flow. It is usually used in wheat, rice, cotton, tea, mulberry, tomatoes, apples, melons, potatoes, forest trees, etc. It is a good plant growth stimulant. If used to prevent fruit drop, the concentration should not be too high, otherwise it will cause the opposite effect, because high concentrations of naphthalene acetic acid can promote the production of ethylene in plants; when used to promote roots, it should be combined with indole acetic acid or other substances that have root-promoting effects. When using a mixture of chemicals, naphthyl acetic acid alone has a good root-promoting effect on crops, but the seedling growth is not ideal. When spraying fruits and melons, it is advisable to spray the leaves evenly. The general amount of spray liquid for field crops is about 7.5kg/100m2. Fruit trees are 11.3~19kg/100m2. Treatment concentration: 10~30mg/Lspray for melons and fruits, 20mg/LSoak seeds for 6~12h, for cotton 10~20mg/LSpray 2 to 3 times during the blooming period.
4. Used in organic synthesis, used in medicine as a raw material for nose and eye net, and also a broad-spectrum plant growth regulator.
rial”>Soak seeds for 6~12h, and soak cotton for 10~20mg/L ;”>Spray 2 to 3 times during the blooming period.
4. Used in organic synthesis, used in medicine as a raw material for Biyanjing, and also a broad-spectrum plant growth regulator.

 微信扫一扫打赏
微信扫一扫打赏

