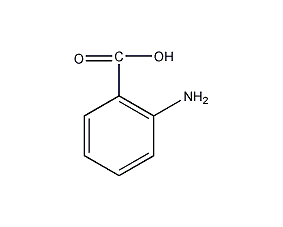
Structural formula
| Business number | 03AC |
|---|---|
| Molecular formula | C7H7NO2 |
| Molecular weight | 137.14 |
| label |
anthranilic acid, 2-aminobenzoic acid, 2-AA, 2-Aminobenzoic acid, o-Aminobenzoic, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:118-92-3
MDL number:MFCD00007712
EINECS number:204-287-5
RTECS number:CB2450000
BRN number:471803
PubChem number:24846741
Physical property data
1. Properties: White to slightly yellow crystalline powder with a sweet taste.
2. Density (g/mL, 25℃): 1.412
3. Relative vapor density (g/mL, air=1): 4.7
4. Melting point (ºC): 144-146
5. Boiling point (ºC, normal pressure): 285
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): 150
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC ): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in alcohol, ether, hot chloroform, hot water, slightly soluble In benzene, insoluble in cold water.
Toxicological data
1. Acute toxicity: Mouse oral LD50: 1400mg/kg
Ecological data
This substance is harmful to the environment and can cause pollution to water bodies and the atmosphere. Organic acids can easily form acid rain during atmospheric chemistry and atmospheric physical changes. Therefore, when the pH value drops below 5, it will cause serious harm to animals and plants, and the reproduction and development of fish will be seriously affected. Metals in the soil and water sediments in the watershed can be dissolved into the water and poison the fish. Acidification of water bodies will also lead to changes in the composition and structure of aquatic organisms. Acid-resistant algae and fungi will increase, while root plants, bacteria and vertebrates will decrease, and the decomposition rate of organic matter will decrease. Acidification will seriously lead to the reduction or death of fish in lakes and rivers.
Molecular structure data
1. Molar refractive index: 37.41
2. Molar volume (cm3/mol): 104.2
3. Isotonic specific volume (90.2K): 295.2
4. Surface tension (dyne/ cm): 64.3
5. Polarizability (10-24cm3): 14.83
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 4
6. Topological molecule polar surface area 63.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 136
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, and strong alkali.
2. Irritating. It is irritating to the eyes, respiratory system and skin. Detailed toxicity has not been reported. Its toxicity and protective methods can be found in m-aminophenol.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
2. Packed in iron barrels (cast bodies), with a net weight of 70kg per barrel. Sun protection and waterproofing should be used during storage and transportation. Place in a cool, ventilated place. Store and transport according to regulations on toxic substances.
Synthesis method
It is obtained by the amidation reaction of phthalic anhydride and ammonia to generate sodium anthranilate, which is then degraded by sodium hypochlorite to generate sodium anthranilate, and finally neutralized. The raw material consumption quota is 1340kg/t of phthalic anhydride (melting point 128-131℃), 6484kg/t of sodium hypochlorite (≥10% available chlorine), 2350kg/t of ammonia (≥16%), and 2924kg/t of liquid alkali (30%).
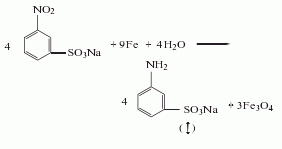
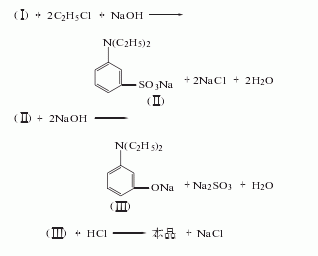
Purpose
1. Intermediates for dyes, medicines, pesticides and spices. In terms of dyes, it is used to make azo dyes, anthraquinone dyes, and indigo dyes. For example, disperse yellow GC, disperse yellow 5G, disperse orange GG, active brown K-B3Y, neutral blue BNL. In medicine, it is used in the manufacture of antiarrhythmic drugs oftenroline and vitamin L, non-steroidal anti-inflammatory analgesics mefenamic acid and yantongjing, non-barbiturate hypnotics methaqualone, and strong tranquillizer Thiel Deng. As a chemical reagent, anthranilic acid can be used as a complex reagent for the determination of cadmium, cobalt, mercury, magnesium, nickel, lead, zinc and cerium. It can be used together with 1-naphthylamine to determine nitrite. This product is also used in other organic synthesis. Using anthranilic acid as raw material, through salt formation, diazotization, reduction and cyclization, 3-Hydroxyindazole (3-Hydroxyindazole) can be obtained. When anthranilic acid is used to produce methyl anthranilate, per ton Product consumption is 925kg. To produce each ton of indigo, 1495kg of anthranilic acid is consumed. Anthranilic acid is moderately toxic and can irritate skin and mucous membranes.
2. Used as complexing reagents and experimental reagents.
3. Used with 1-naphthylamine to determine nitrite, organic synthesis, pharmaceutical industry, and dye synthesis.

 微信扫一扫打赏
微信扫一扫打赏

