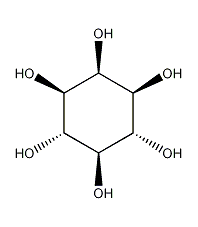
Structural formula
| Business number | 01YR |
|---|---|
| Molecular formula | C6H12O6 |
| Molecular weight | 180.16 |
| label |
Inositol (fibrosaccharide), Hexahydroxycyclohexane, cyclohexamethanol, Cyclohexanol, muscle sugar, lactogen, luteal nutrients, prolactin, (cis)-1,2,3,5-(trans)-4,6-cyclohexahexanol, i-Inositol, Meso-Inositol, Lactogenic hormone, Luteotrophic hormone, (cis)-1,2,3,5-(trans)-4,6-Cyclohexanehexol, nutritional additives, alcohol solvent |
Numbering system
CAS number:87-89-8
MDL number:MFCD00077932
EINECS number:201-781-2
RTECS number:NM7520800
BRN number:1907329
PubChem number:24895982
Physical property data
1. Properties: It is a white crystalline powder that does not contain crystal water and is non-hygroscopic. Those containing two molecules of crystal water are weathered crystals and lose water at 100°C. Odorless and sweet. Stable in air.
2. Density (g/mL, 20/4℃): 1.752
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 225-227℃ (anhydrous), 218℃ (dihydrate)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC ): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined Determined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in water, insoluble in absolute ethanol, ether and chloroform, its aqueous solution is neutral.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 37.21
2. Molar volume (cm3/mol): 88.3
3. Isotonic specific volume (90.2K ): 280.5
4. Surface tension (dyne/cm): 101.6
5. Polarizability (10-24cm3): 14.75
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): -3.7
2. Number of hydrogen bond donors: 6
3. Number of hydrogen bond acceptors: 6
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 121
p>
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 104
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable in air. Aqueous solutions are neutral to litmus.
Storage method
Seal and store in a cool, ventilated and dry place.
Synthesis method
1. Obtained from hydrolysis and neutralization of Fetin. Put the phytin and water into a high-pressure hydrolysis pot, heat it in an airtight manner, gradually increase the internal pressure to about 0.5MPa, and stir for 8 hours. Check the pH value of the material, 2.5-3.0 is the end point of the reaction. Put the hydrolyzate into a neutralization pot, neutralize it with lime milk, keep the temperature at 70-80°C, and control the pH value at 7-8. Use a centrifuge to throw out the filtrate, and the filter residue can be used as fertilizer (calcium phosphate). Heat the above neutralized filtrate to 80-90°C, add 0.5-0.7% activated carbon, and stir continuously. Use a sand core to perform suction filtration for about 20 minutes. The filtered clear liquid is sucked into the concentration pot and concentrated at 90°C for 4-5 hours. When the relative density reaches 1.24-1.28, the material can be discharged and placed in an enamel bucket or stainless steel bucket for cooling. When crystals precipitate, use manual stirring, cool to 20-25°C, and send it to a centrifuge to spin dry to obtain crude yellow inositol. The crude mother liquor can be thrown out to concentrate and crystallize again. Add water or linear solution to the crude product, heat to dissolve, add activated carbon for decolorization, then add barium oxyoxide solution and ammonium oxalate solution to precipitate and remove sulfate and calcium ions respectively. Filter while it is hot, cool the filtrate to 30°C, crystallize, and filter (the same as the mother liquor). Wash the filter cake with a small amount of ethanol, dry it at 80°C for 6 hours, and finally pass it through an 18-mesh sieve to obtain myo-inositol.
2.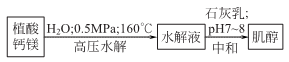
Dry calcium phytate and water are beaten in a ratio of 1: (3~3.5). Calcium phytate paste can be directly hydrolyzed with water. Atmospheric pressure hydrolysis requires the addition of a catalyst, the hydrolysis cycle is long, and the yield of myo-inositol is low. Pressurized hydrolysis can increase the yield and shorten the production cycle. After the hydrolysis is completed, lime milk can be added to convert the soluble phosphates and phosphate acid salts into insoluble salts and remove them. Add lime milk to neutralize to pH 8~9 and then filter. Then add 1.5% activated carbon, boil and keep warm for 15 to 20 minutes to remove pigments and impurities. The decolorized liquid is then crystallized at 32°C to obtain crude inositol. The crude product can be washed with distilled water to remove inorganic acids and inorganic salts. The more advanced method is to use anion and cation exchange resins to remove inorganic acids and inorganic salts, which avoids the loss of myo-inositol caused by water washing, and the product quality is also better. The refined myo-inositol can be dried at 60-70℃ to obtain the finished product.
Purpose
1. This product is one of the multivitamins B, which can promote cell metabolism, improve cell nutrition, promote development, increase appetite, and restore physical strength. It can prevent the accumulation of fat in the liver, accelerate the removal of excess fat in the heart, and has a synergistic lipotropic effect with choline, so it is used to treat excess liver fat and cirrhosis. According to the “Hygienic Standards for the Use of Food Nutritional Enhancers (1993) promulgated by the Ministry of Health of my country, it can be used in infant foods and fortified beverages, and the dosage is 380-790mg/kg. Vitamin drugs and hypolipidemic drugs promote fat metabolism in the liver and other tissues, and are used as auxiliary treatments for fatty liver and hyperlipidemia. Widely used in food and beverage additives.
2.Diagnosis and treatment of hematocerebral infertility, secondary amenorrhea and individual normal women caused by long-term use of steroidal contraceptives Amenorrhea etc.

 微信扫一扫打赏
微信扫一扫打赏

