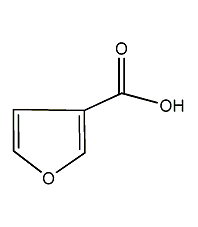
Structural formula
| Business number | 01Z2 |
|---|---|
| Molecular formula | C5H4O3 |
| Molecular weight | 112.08 |
| label |
2-furoic acid, a-furoic acid, 2-Furoic acid, a-Furoic acid, synthetic raw materials, Intermediates |
Numbering system
CAS number:88-14-2
MDL number:MFCD00003238
EINECS number:201-803-0
RTECS number:LV1763000
BRN number:110149
PubChem number:24894811
Physical property data
1. Properties: White monoclinic rhombus crystal, sublimates at 130-140℃ (6.67~8.0kPa).
2. Density (g/mL, 25/4℃): 1.322
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 133~134
5. Boiling point (ºC, 101.3kpa): 230~232
6. Boiling point (ºC, 2.67kpa): 141~144
7. Refractive index: Undetermined
8. Flash point (ºC): 137
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined 19. Solubility: Easily soluble in ethanol and ether, partially soluble in water: 26ml cold water (15℃ ) or 1g of furoic acid soluble in 4ml of boiling water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 25.48
2. Molar volume (cm3/mol): 84.7
3. Isotonic specific volume (90.2K ): 223.3
4. Surface tension (dyne/cm): 48.2
5. Polarizability (10-24cm3): 10.10
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0.5
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
p>
6. Topological molecular polar surface area (TPSA): 50.4
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 99.8
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain atoms Number of stereocenters: 0
13. Number of determined stereocenters of chemical bonds: 0
14. Number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
1. Obtained from oxidation of furfural by air. The reaction was carried out at 45°C with vigorous stirring. The reactants are filtered to recover the catalyst copper oxide, and the filtrate is acidified with 50% sulfuric acid to a pH of 3-2.5, filtered with suction, washed with water, and dried to obtain furoic acid. Furfural can also be prepared by oxidizing furfural with sodium hypochlorite or potassium permanganate for 2 hours in a neutral or alkaline medium, and then acidifying it with hydrochloric acid, with a yield of up to 98%.
2. Tobacco: OR, 44; FC, 9; OR, 26; BU, 26.
Purpose
1. Furoic acid is used to synthesize methylfuran, furfuramide and furoate esters and salts. In the plastics industry, it can be used as plasticizer, thermosetting resin, etc. It is used as a preservative and bactericide in the food industry, as well as a coating additive and medicine. Intermediates for spices, etc.
2. Used in the synthesis of fururacil penicillin and cephalothin, etc.

 微信扫一扫打赏
微信扫一扫打赏

