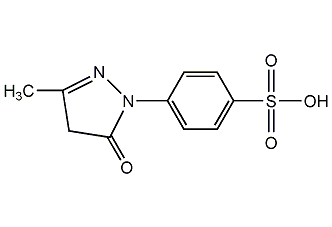
Structural formula
| Business number | 020P |
|---|---|
| Molecular formula | C10H10N2O4S |
| Molecular weight | 254.26 |
| label |
1-(4-phenylsulfonate)-3-methyl-5-pyrazolone, 1-(4-Sulfophenyl)-3-methyl-5-pyrazolone, 4-(3-Methyl-5-oxo-2-pyrazolin-1-yl)benzenesulfonic acid, Heterocyclic compounds |
Numbering system
CAS number:89-36-1
MDL number:MFCD00020756
EINECS number:201-901-3
RTECS number:None
BRN number:619424
PubChem number:24848135
Physical property data
1. Physical properties:White needle-like or light yellow crystal
2. Melting point: 290~320℃ (decomposition).
3. Solubility: Slightly soluble in hot water, slightly soluble in cold water, soluble in liquid alkali, hardly soluble in ethanol , acetic acid, ether.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 61.81
2. Molar volume (cm3/mol): 166.1
3. Isotonic specific volume (90.2K ): 469.6
4. Surface tension (dyne/cm): 63.9
5. Polarizability (10-24cm3): 24.50
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 0
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecular polar surface area (TPSA): 87
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 443
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
See 1-phenyl-3-methyl-5-pyrazol(lin)one.
Storage method
Lined with plastic bags and packed in iron drums, 100kg per bag. Store in a cool, ventilated, dry warehouse away from sunlight, heat and moisture. When transporting, load and unload gently to prevent damage to the packaging.
Synthesis method
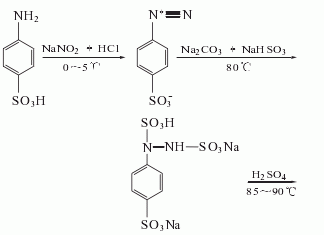
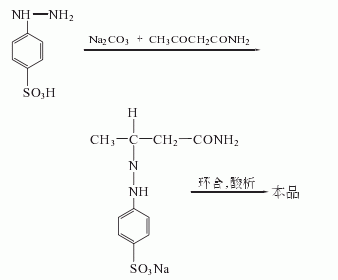
After diazotization with p-aminobenzene sulfonic acid, sodium nitrite and hydrochloric acid,Reduction with sodium bisulfite and sodium carbonate, hydrolysis with sulfuric acid to obtain p-sulfophenylhydrazine, condensation, cyclization and acid precipitation with acetyl acetamide to obtain pyrazolone.
Purpose
Intermediate for manufacturing acid yellow G, weak acid yellow G and other dyes.

 微信扫一扫打赏
微信扫一扫打赏

