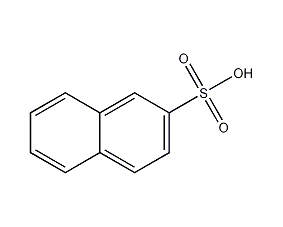
Structural formula
| Business number | 03BS |
|---|---|
| Molecular formula | C10H8O3S |
| Molecular weight | 208.23 |
| label |
β-Naphthalenesulfonic acid, Naphthalene-2-sulfonic acid, β-Naphthalene sulfonic acid, diffusing agent, aromatic sulfur compounds |
Numbering system
CAS number:120-18-3
MDL number:MFCD00004089
EINECS number:204-375-3
RTECS number:QK1225000
BRN number:None
PubChem number:24855002
Physical property data
1. Character: White to slightly brown leaf-like crystals
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density ( g/mL, air=1): Undetermined
4. Melting point (ºC): 91 (anhydrous), 83 (trihydrate), 124 (monohydrate)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, KPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper explosion limit (%, V/V): Undetermined
18. The lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water, alcohol and ether. Easy to deliquesce.
Toxicological data
1. Acute toxicity: Rat oral LD50: 4440mg/kg
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 54.65
2. Molar volume (cm3/mol): 146.3
3. Isotonic specific volume (90.2K): 405.1
4. Surface tension (dyne/cm): 58.7
5. Polarizability (10-24cm3): 21.66
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 62.8
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 291
10. Number of isotope atoms: 0
11. Determine the atomic stereocenter Quantity: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain chemical bonds Number of stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Toxic. Orally administered LD50440 mg/kg to rats. Flush skin contamination with water and wash thoroughly with soap.
Storage method
Brown glass bottles are lined with fiberboard or calcium plastic box lining. Store in a cool, ventilated warehouse. Keep away from heat sources and fire. Store and transport isolated from oxidants.
Synthesis method
Obtained from sulfonation of naphthalene. It is obtained by using naphthalene as raw material and 98% sulfuric acid as sulfonating agent at 160-166℃. During the sulfonation process, a small amount of α-naphthalene sulfonic acid is generated as a by-product. By heating, the unstable α-naphthalene sulfonic acid can be hydrolyzed and detached from the sulfonic acid group at 140-150°C to become naphthalene and sulfuric acid, and use water vapor to The naphthalene can be blown out. When hydrolyzing naphthalene, a small amount of alkali solution should be added to neutralize part of the sulfuric acid. It also reacts with naphthalene sulfonic acid to form β-naphthalene sulfonic acid sodium salt crystals, which can be filtered to obtain the finished product.
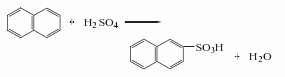
p>
Purpose
Used to produce 2-naphthol, 2-naphtholsulfonic acid, 2-naphthylamine sulfonic acid and other dye intermediates. Diffusion agent N can be obtained by condensing this product with formaldehyde

 微信扫一扫打赏
微信扫一扫打赏

