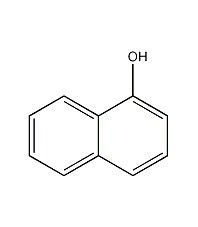
Structural formula
| Business number | 0220 |
|---|---|
| Molecular formula | C10H8O |
| Molecular weight | 144 |
| label |
alpha-naphthol, 1-Hydroxynaphthalene, α-Naphthol, 1-Hydroxynaphthalene, 1-Naphthol |
Numbering system
CAS number:90-15-3
MDL number:MFCD00003930
EINECS number:201-969-4
RTECS number:QL2800000
BRN number:1817321
PubChem number:24897451
Physical property data
1. Properties: colorless or yellow, with phenol smell, crystal or powder, turns rose color when exposed to light.
2. Relative density (d994) : 1.0989
3. Relative vapor density (g/mL, air =1): Undetermined
4. Melting point (ºC): 96
5. Boiling point (ºC, normal pressure): 278~280
6. Standard heat of combustion (enthalpy) of the crystal phase (kJ·mol-1): -4957.4
7. Refractive index(n99 D): 1.6224
8. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5048.6 p>
9. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -29.9
10. The crystal phase standard claims heat (enthalpy) (kJ ·mol-1): -121.0
11. Vapor pressure (kPa, 94ºC): 0.13kPa
12. Saturated vapor pressure (kPa, 60ºC ): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical Pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water, easily soluble in benzene, ethanol, ether, and chloroform and alkaline solutions, etc.
20. Flash point (ºC): 125
Toxicological data
Toxic, rat oral LD50: 2590mg/kg.
Ecological data
None
Molecular structure data
1. Molar refractive index 45.97
2. Molar volume (cm3/mol): 121.9
3. Isotonic specific volume (90.2K) : 326.1
4. Surface tension (dyne/cm): 51.0
5. Polarizability (10-24cm3): 18.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. TotalNumber of price key units: 1
Properties and stability
1. Exist in smoke.
2. Can sublimate and evaporate with steam.
3. Swallowing or absorption through the skin can cause poisoning. It is irritating to the eyes and mucous membranes and can cause rashes at the contact parts of the human body. The symptoms are vomiting, diarrhea, abdominal pain, anemia, collapse, and protein precipitation. Production equipment must be sealed to prevent leakage, and any spills on the skin must be washed immediately. The workshop should be ventilated and operators should wear protective equipment.
Storage method
This product should be stored in a sealed, cool, dry place.
Synthesis method
1. There are many production methods for 1-naphthol, which can be divided into: α-naphthalene sulfonic acid alkali fusion method, α-naphthylamine hydrolysis method, and tetralin method. 1. α-naphthylamine hydrolysis method: α-naphthylamine is hydrolyzed using dilute sulfuric acid as a medium to obtain α-naphthol crude product. The crude product is separated, neutralized, and distilled to obtain the finished product. 2. In the sulfonation method, refined naphthalene is sulfonated with sulfuric acid at low temperature to obtain α-naphthalene sulfonic acid, and β-naphthalene sulfonic acid is produced as a by-product. After the by-product is separated, it is neutralized, alkali fused, acidified, and then separated and distilled to obtain the finished product. . Raw material consumption quota: sulfuric acid (98%) 900kg/t, refined naphthalene (99.5%) 1150kg/t, caustic soda (42%) 1300kg/t.
2. The aqueous solution reacts with ferric chloride to form purple precipitate.
3. It is prepared from 1-naphthalenesulfonic acid through alkaline solution.
Purpose
1. The demand for 1-naphthol is only about 5% of that of 2-naphthol. However, 1-naphthol, as a raw material for the pesticide carbaryl, is in great demand. Intermediates derived from 1-naphthol include N-phenyl-1-naphthylamine, N-ethyl-1-naphthylamine, N-cyclohexyl-1-naphthylamine, and 1-hydroxy-2-naphthylcarboxylic acid. , 1-naphthol-2-sulfonic acid, 1-naphthol-4-sulfonic acid, 1,7-Klebsenic acid, acetamido-7-naphthol, 2,4-dinitronaphthol, etc. In the dye industry, land is used to produce acid yellow 1, acid orange 20, mordant black 3, mordant black 11, solvent red 3, solvent orange 5, oil palm GB, etc. In the pharmaceutical industry, it can be used to manufacture preservatives and anti-mild rheumatism drugs. It is also an effective antioxidant for many aldehydes, mineral oils and vegetable oils, and is widely used in synthetic fragrances, rubber antioxidants and color couplers of film films.
2. It is the basic raw material for organic synthesis industry, pharmaceutical industry and dye industry. It is also used in the production of plastics, rubber antioxidants, color film couplers, etc. It is a restricted substance in cosmetic components. The maximum allowable content is 0.5%. The allowable use range is coupling hair dye for hair dyeing.

 微信扫一扫打赏
微信扫一扫打赏

