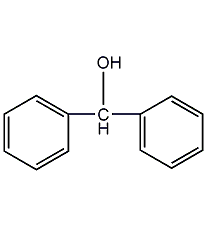
Structural formula
| Business number | 022Z |
|---|---|
| Molecular formula | C13H12O |
| Molecular weight | 184.23 |
| label |
benzyl alcohol, α-Phenylbenzyl alcohol, Diphenylmethanol, Diphenyl carbinol, (C6H5)2CHOH |
Numbering system
CAS number:91-01-0
MDL number:MFCD00004488
EINECS number:202-033-8
RTECS number:DC7452000
BRN number:1424379
PubChem number:24866820
Physical property data
1. Properties: Colorless needle-like crystals.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 69
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 99.84kPa): 298
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol, ether, chloroform and carbon disulfide, At 20°C, 1g of product is soluble in 2000ml of water and almost insoluble in cold crude gasoline.
Toxicological data
Acute toxicity:
Oral LD50 5mg/kg(rat)
Skin LD50 >5mg/kg(rbt)
Skin irritation mild unknown mg ( rbt)
Eye irritation mild unknown mg (rbt)
Main irritating effects:
On skin: May cause inflammation
On eyes: May cause inflammation
Sensitization: May cause sensitization through skin contact
Ecological data
General remarks
Water hazard class 1 (German regulations) (self-assessment via list) The substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.��
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 57.10
2. Molar volume (cm3/mol): 167.0
3. Isotonic specific volume (90.2K ): 433.7
4. Surface tension (dyne/cm): 45.3
5. Polarizability (10-24cm3): 22.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 137
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product should be sealed and stored in a cool place.
Storage method
None
Synthesis method
1. Preparation method:
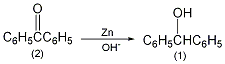
In a 1L reaction bottle equipped with a stirrer, thermometer and reflux condenser, add 50g of benzophenone (2) (0.275moPl), 500mL (95%) of ethanol, 50g of sodium hydroxide and 50g of zinc powder (0.76mol ), stir vigorously, and slowly raise the temperature to about 70°C. After 3 hours, lower the temperature, filter, and wash the filter residue twice with hot 95% ethanol (the remaining zinc powder cannot become dry to avoid catching fire). Pour the filtrate into 2L ice water and acidify it with 100mL concentrated hydrochloric acid to precipitate a white solid. After suction filtration and drying in the air, 49g of crude product benzyl alcohol was obtained, mp65°C. Recrystallize with 50 mL of hot ethanol, cool in an ice-salt bath, filter the precipitated colorless solid, and dry to obtain pure benzyl alcohol ①(1) 36g, mp68°C. Some products can be recovered from the mother liquor. Note: ① Benzophenone can also be prepared by reducing benzophenone with sodium borohydride in 95% ethanol, with a yield of about 90%. [1]
Purpose
Used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

