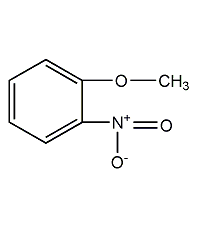
Structural formula
| Business number | 023D |
|---|---|
| Molecular formula | C7H7NO3 |
| Molecular weight | 153.14 |
| label |
1-methoxy-2-nitrobenzene, o-Methoxynitrobenzene, o-nitroanisole, o-Nitroanisole, 1-Methoxy-2-nitrobenzene, o-Nitroanisole, O2NC6H4OCH3, detergent, pesticides, Multifunctional solvent |
Numbering system
CAS number:91-23-6
MDL number:MFCD00007096
EINECS number:202-052-1
RTECS number:BZ8790000
BRN number:1868032
PubChem number:24854160
Physical property data
1. Properties: colorless crystal or reddish liquid [1]
2. Melting point (℃): 9.6[2]
3. Boiling point (℃): 273[3]
4. Relative density (water=1): 1.26[4]
5. Saturated vapor pressure (kPa): 0.53 (144°C) [5]
6. Critical pressure (MPa): 3.76[6]
7. Octanol/water partition coefficient: 1.73[7]
8. Flash point (℃) :>112[8]
9. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol and ether. [9]
Toxicological data
1. Acute toxicity[10] LD50: 740mg/kg (oral in rats); 1300mg/kg (oral in mice)
2. Irritation No data available
3. Mutagenicity[11] Microbial mutagenicity: rats Salmonella typhi 666μg/dish. DNA repair: Bacillus subtilis 500nL/dish. Bacterial genetic analysis: Hamster ovary 1060mg/L. Sister chromatid exchange: Hamster ovary 123mg/L.
4. Carcinogenicity [12] IARC Carcinogenicity Comment: G2B, suspected human carcinogen.
Ecological data
1. Ecotoxicity[13] LC50: 25mg/L (48h) (medaka)
2. Biodegradation Properties No data available
3. Non-biodegradability No data available
Molecular structure data
1. Molar refractive index: 39.47
2. Molar volume (cm3/mol): 125.2
3. Isotonic specific volume (90.2K ): 319.4
4. Surface tension (dyne/cm): 42.2
5. Polarizability (10-24cm3): 15.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecular extremes�Surface area 55
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 143
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13 .Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties When heated under pressure with ammonia, o-nitroaniline is generated. It is heated under pressure together with hydrazine hydrate to generate 1-hydroxybenzenetriazole.
2. Stability[14] Stable
3. Incompatible substances[15] Strong oxidants, strong reducing agents, strong acids, strong bases
4. Conditions to avoid contact [16] Heating
5. Polymerization hazard[17] No polymerization
6. Decomposition products[18] Nitrogen oxides
Storage method
Storage Precautions[19] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Methoxylation reaction between o-nitrochlorobenzene, caustic soda and methanol is carried out to obtain a crude product. The crude product is distilled, washed and dried to obtain the finished product. Raw material consumption quota: o-nitrochlorobenzene 1120kg/t, methanol 270kg/t.
Refining method: Repeated vacuum distillation in the absence of air.
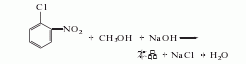
Purpose
1. This product is used in industries such as dyes, medicines, and spices. It can be used to produce anthranilate, dianisidine, naphthyl AS-OL, big red base B, direct lake blue 6B, and reactive soluble blue. KD-7G, detergent LS, etc. It has insecticidal effect.
2. Used in organic synthesis, manufacturing dyes and drugs. [20]

 微信扫一扫打赏
微信扫一扫打赏

