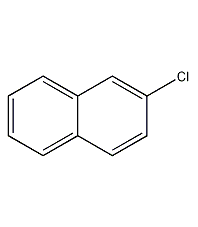
Structural formula
| Business number | 023Q |
|---|---|
| Molecular formula | C10H7Cl |
| Molecular weight | 162.62 |
| label |
β-Chloronaphthalene, β-Chloronaphthalene, high boiling point solvents, grease solvent, Gas Chromatography Stationary Solution |
Numbering system
CAS number:91-58-7
MDL number:MFCD00035731
EINECS number:202-079-9
RTECS number:QJ2275000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: flake crystal, can be sublimated.
2. Boiling point (ºC, 101.3kPa): 259
3. Boiling point (ºC, 1.33kPa): 118
4. Boiling point (ºC, 2.67 kPa): 132.6
5. Boiling point (ºC, 8.00kPa): 161.2
6. Melting point (ºC): 59.8
7. Relative density (g /mL, 20/4ºC): 1.178
8. Relative density (g/mL, 16/4ºC): 1.2656
9. Relative density (g/mL, 80/4ºC ): 1.1297
10. Refractive index (70.7ºC): 1.6079
11. Vapor pressure (kPa, 25ºC): 256
12. Solubility: Insoluble in water, soluble in ethanol, ether, benzene, chloroform and carbon disulfide.
13. Refractive index at room temperature (n20): 1.607913
14. Relative density (25℃, 4℃ ): 1.138071
15. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): 55.4
16. The gas phase standard claims heat (enthalpy) (kJ·mol-1): 137.4
Toxicological data
Rat oral LD50: 2078mg/kg. Mouse oral LD50: 886mg/kg.
Ecological data
None
Molecular structure data
1. Molar refractive index: 48.99
2. Molar volume (cm3/mol): 135.5
3. Isotonic specific volume (90.2K ): 346.9
4. Surface tension (dyne/cm): 42.9
5. Polarizability (10-24cm3): 19.42
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine ���Number of stereocenters of chemical bonds: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
It has good chemical stability, is not easy to burn, and can undergo halogenation, nitration, sulfonation and chloromethylation reactions. It is hydrolyzed in sodium hydroxide aqueous solution at 260~300℃ to generate β-naphthol.
Storage method
Should be sealed and stored in a cool place
Synthesis method
None
Purpose
High boiling point solvents, grease solvents, gas chromatography stationary solutions. Used in organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

