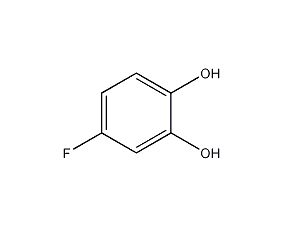
Structural formula
| Business number | 04GQ |
|---|---|
| Molecular formula | C6H5FO2 |
| Molecular weight | 128.10 |
| label |
heterocyclic compounds, Pharmaceuticals, pesticide intermediates |
Numbering system
CAS number:367-32-8
MDL number:None
EINECS number:None
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
一 , physical property data
Traits :Not available
Density (g/mL,25/4℃): Not available
Relative Vapor density (g/mL, air=1):Not available
Melting point (ºC): 90-91
Boiling point (ºC, normal pressure): Not available
Boiling point (ºC, 5.2kPa): Not available
Refraction Rate: Not available
Flash Point (ºC): Not available
Optical rotation (º): Not available
Spontaneous combustion Point or ignition temperature (ºC): Not available
Steam Pressure (kPa, 25ºC): Not available
saturated Vapor pressure (kPa, 60ºC): Not available
Burn Heat (KJ/mol):Not available
Critical Temperature (ºC): Not available
Critical Pressure (KPa): Not available
oil and water Log value of the (octanol/water) partition coefficient:Not available
Explosion Upper limit (%, V/V): Not available
Explosion Lower limit (%, V/V): Not available
Dissolve Properties: Not available
Toxicological data
Two , Toxicological data:
Acute Toxicity:Not available .
Ecological data
Three , Ecological data:
1 ,Other harmful effects: This substance may be harmful to the environment, and special treatment should be given to water bodies. Notice.
Molecular structure data
1. Molar refractive index: 30.00
2. Molar volume (m3/mol):90.5
3. isotonic specific volume (90.2K):244.4
4. Surface Tension (dyne/cm):53.2
Acute Toxicity:Not available .
Ecological data
Three , Ecological data:
1 ,Other harmful effects: This substance may be harmful to the environment, and special treatment should be given to water bodies. Notice.
Molecular structure data
1. Molar refractive index: 30.00
2. Molar volume (m3/mol):90.5
3. isotonic specific volume (90.2K):244.4
4. Surface Tension (dyne/cm):53.2
5. Polarizability(10-24cm3):11.89
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 8
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 97.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Using p-fluorophenol as raw material, under the catalysis of concentrated sulfuric acid, the acetic anhydride esterification reaction produces p-fluorophenyl acetate. ; p-Fluorophenyl acetate occurs under the action of aluminum trifluorideRearrangement reaction, generate5-fluorine-2-Hydroxyacetophenone; reagentDakinSynthesized by oxidation4-Fluorocatechol.
Purpose
This product is mainly used in the production of pesticides and pharmaceutical intermediates.
: auto; mso-list: l0 level2 lfo1; tab-stops: list 36.0pt” align=left>5. Polarizability(10-24cm3):11.89
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 8
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 97.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Using p-fluorophenol as raw material, under the catalysis of concentrated sulfuric acid, the acetic anhydride esterification reaction produces p-fluorophenyl acetate. ; p-Fluorophenyl acetate occurs under the action of aluminum trifluorideRearrangement reaction, generate5-fluorine-2-Hydroxyacetophenone; reagentDakinSynthesized by oxidation4-Fluorocatechol.
Purpose
This product is mainly used in the production of pesticides and pharmaceutical intermediates.

 微信扫一扫打赏
微信扫一扫打赏

