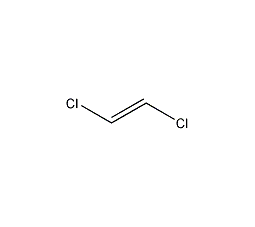
Structural formula
| Business number | 03ZT |
|---|---|
| Molecular formula | C2H2Cl2 |
| Molecular weight | 96.94 |
| label |
None yet |
Numbering system
CAS number:156-60-5
MDL number:MFCD00062942
EINECS number:205-860-2
RTECS number:KV9400000
BRN number:1420761
PubChem number:24872050
Physical property data
1. Properties: Colorless liquid with an odor similar to chloroform.
2. Boiling point (ºC, 101.3kPa): 47.7
3. Melting point (ºC): -49.4
4. Relative density (g/mL, 20/4ºC): 1.2565
5. Refractive index (n20ºC): 1.4457
6. Viscosity (mPa·s, gas): 0.404
7. Flash point (ºC, open): 3.9
8. Heat of evaporation (KJ/mol, b.p.): 28.91
9. Heat of fusion (KJ/mol): 11.99
10. Heat of combustion (KJ/mol, 18.7ºC): 1095.39
11. Specific heat capacity (KJ/(kg·K), 20ºC): 1.16
12. Critical temperature (ºC): 243.3
13. Critical pressure (MPa): 5.53
14. Volume expansion coefficient (K-1, 15~45ºC ): 0.00136
15. Solubility: Slightly soluble in water. Miscible with various organic solvents such as ethanol and ether.
16. Relative density (25℃, 4℃): 1.2502
17. Refractive index at room temperature (n25): 1.4435
18. Solubility parameter (J·cm-3)0.5: 18.549
19. van der Waals area (cm2 ·mol-1): 5.760×109
20. van der Waals volume (cm3·mol-1): 40.180
21. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -0.4
22. Gas phase standard entropy (J·mol-1·K-1): 289.96
23. Gas phase standard generation is free Energy (kJ·mol-1): 22.0
24. Gas phase standard hot melt (J·mol-1·K-1 ): 66.55
25. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -29.7
26. Liquid phase standard hot melt (J·mol-1·K-1): 118.4
Toxicological data
Low toxicity, LD50 (rat, oral) 1ml/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 21.07
2. Molar volume (cm3/mol): 77.9
3. Isotonic specific volume (90.2K ): 175.8
4. Surface tension��dyne/cm): 25.9
5. Dielectric constant (F/m): Not available
6. Polarizability (10-24 cm3): 8.35
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 19.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It has a spicy smell. It gradually decomposes when exposed to air, light and moisture to form hydrogen chloride. It is irritating and anesthetic in high concentrations.
Storage method
It should be sealed and stored in a dry place at 4°C away from light.
Synthesis method
None yet
Purpose
Solvent of fat, phenol, camphor, etc. Organic Synthesis. Dyes, fragrances, paints, thermoplastics. Degreaser.

 微信扫一扫打赏
微信扫一扫打赏

