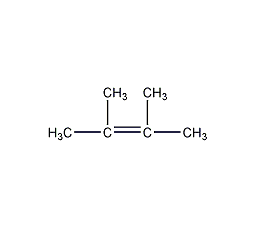
Structural formula
| Business number | 05T1 |
|---|---|
| Molecular formula | C6H12 |
| Molecular weight | 84 |
| label |
None yet |
Numbering system
CAS number:563-79-1
MDL number:MFCD00008897
EINECS number:209-263-8
RTECS number:None
BRN number:1361357
PubChem number:24853167
Physical property data
1. Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25/4℃): 0.7080
3. Refractive index (nD20): 1.4122
4. Flash point (℃): -16
5. Melting point (℃): -74.28
6. Boiling point (ºC): 73.2
7. Solubility: Soluble in ethanol, ether, acetone and chloroform, insoluble in water.
8. Solubility parameter (J·cm-3)0.5: 15.867
9. van der Waals area ( cm2·mol-1): 9.700×109
10. van der Waals volume (cm3·mol-1): 64.700
11. Gas phase standard combustion heat (enthalpy) (kJ·mol-1) : -4006.26
12. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -69.79
13. Gas phase standard entropy (J ·mol-1·K-1): 364.64
14. Gas phase standard free energy of formation (kJ·mol-1): 65.35
15. Gas phase standard hot melt (J·mol-1·K-1): 123.51
16. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3973.63
17. Liquid phase standard claimed heat (enthalpy) (kJ·mol -1): -102.42
18. Liquid phase standard entropy (J·mol-1·K-1 ): 270.20
19. Liquid phase standard free energy of formation (kJ·mol-1): 60.86
20. Liquid phase standard hot melt (J ·mol-1·K-1): 174.68
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 29.58
2.Molar volume (cm3/mol): 120.7
3. Isotonic volume (90.2K): 250.4
4. Surface tension (dyne/cm) : 18.4
5. Polarizability (10-24cm3): 11.73
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 50.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
Flammable. Irritating.
Storage method
2. Storage
Should be stored in a cool and sealed place.
Synthesis method
Synthetic method:
Tetramethylethylene is obtained by dimerization of propylene. Put propylene into the dimerization kettle, add a homogeneous catalyst, and perform a catalytic polymerization reaction under stirring. When the kettle When the internal pressure drops below 0.01MPa, the reaction of the material will be stopped, and the oil layer will be crudely evaporated to remove the trimerization and tetramerization products, namely C9~C19. For olefins, collect the fraction at 53-75°C as a crude product, mainly 2,3-dimethylbutene-1, and then perform catalytic isomerization. The reaction temperature is controlled at 85-90°C, so that the product is converted into 2 , 3-dimethylbutene-2, that is, tetramethylbutene-2, is further distilled, and the 71-74°C fraction is collected, which is the finished product 2,3-dimethylbutene-2, and the rest is Other by-products are C6alkenes. The single-pass conversion rate of propylene in the dimerization reaction is >90%, the dimerization selectivity is 80%, and the isomerization conversion rate is 79%.
Purpose
3. Purpose
Gas chromatography analysis standard. Organic Synthesis.

 微信扫一扫打赏
微信扫一扫打赏

