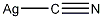
Structural formula
| Business number | 059L |
|---|---|
| Molecular formula | AgCN |
| Molecular weight | 133.90 |
| label |
Precious metal plating reagents and additives |
Numbering system
CAS number:506-64-9
MDL number:MFCD00003409
EINECS number:208-048-6
RTECS number:VW3850000
BRN number:4360924
PubChem number:24867451
Physical property data
1. Properties: White powder or light gray powder, odorless and tasteless, turns brown when exposed to light. [1]
2. Melting point (℃): 320 (decomposition) [2]
3. Relative density ( Water = 1): 3.95[3]
4. Solubility: insoluble in water, insoluble in alcohol, soluble in ammonia, potassium iodide, hot dilute nitric acid. [4]
Toxicological data
1. Skin/eye irritation data:
Standard Draize test rabbit direct contact with skin: 500mg/24HREACTION SEVERITY: Mild;
Standard Draize test rabbit direct contact with eyes: 5mg /24HREACTION SEVERITY: Severe;
2. Acute toxicity: Rat oral LD50: 123mg/kg, no details except lethal dose;
3. Acute toxicity [5] LD50: 123mg/kg (rat oral)
4. Irritation [6]
Rabbit transdermal: 500mg (24h), mild stimulation.
Rabbit eye: 20mg (24h), severe irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[7] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: not available
2. Molar volume (cm3/mol): not available
3. etc. Zhang specific volume (90.2K): None available
4. Surface tension (dyne/cm): None available
5. Dielectric constant: None available
6. Polarizability (10-24cm3): None available
7. Single isotope mass: 132.908167 Da
8. Nominal mass: 133 Da
9. Average mass: 133.8856 Da
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 10
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Stability[8] Stable
2. Incompatible materials[9] Strong oxidants, strong acids
3. Conditions to avoid contact[10] Humid air, light
4. Polymerization hazard[11] No polymerization
5 .Decomposition products[12] Cyanide
Storage method
Storage Precautions[13] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Metallic silver method
Dissolve the metal silver in nitric acid first, then dilute it with water, and add sodium cyanide with constant stirring until the precipitation is complete (keep the solution weak throughout the reaction process Acidic), and then the precipitate is separated, washed, and dried to obtain silver cyanide. The reaction formula is as follows:

2. Metathesis method
In a cold saturated solution of silver nitrate, concentrated potassium cyanide solution and hydrocyanic acid undergo a metathesis reaction to obtain silver cyanide precipitate, which is then separated, washed, and dried to obtain silver cyanide. Finished product. The reaction formula is as follows: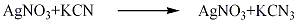
3. Add saturated nitric acid The silver solution is mixed with a measured amount of 78% potassium cyanide solution and filtered quickly. The precipitated silver cyanide was recrystallized twice and dried at 140°C for 4 days.
Purpose
1. Used in medicine, silver plating, protective coatings, autoclave linings, electrical contacts, and electroplating of aircraft engine bearings.
2. Use ether chlorine and alcohol as a base when preparing sterically hindered esters. Preparation of isonitrile and isonitrile. Catalyst for the synthesis of certain heterocycles.
3. Used in medicine and silver plating. [14]

 微信扫一扫打赏
微信扫一扫打赏

