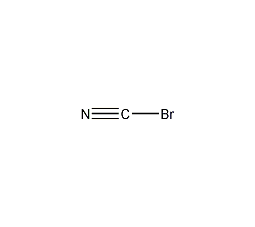
Structural formula
| Business number | 059N |
|---|---|
| Molecular formula | BrCN |
| Molecular weight | 105.93 |
| label |
cyanogen bromide, cyanogen bromide, bromine cyanide, Bromine cyanide, Fungicide |
Numbering system
CAS number:506-68-3
MDL number:MFCD00011597
EINECS number:208-051-2
RTECS number:None
BRN number:1697296
PubChem number:24871758
Physical property data
1. Properties: Colorless or white needle-like or cubic crystals, volatile at room temperature. [8]
2. Melting point (℃): 52[9]
3. Boiling point (℃): 61.4 [10]
4. Relative density (water = 1): 2.02 (20℃) [11]
5 .Relative vapor density (air=1): 3.65[12]
6. Saturated vapor pressure (kPa): 13.33 (23℃)[13] sup>
7. Octanol/water partition coefficient: -0.29[14]
8. Solubility: soluble in water, easily soluble in ethanol , diethyl ether, soluble in benzene. [15]
Toxicological data
1. Acute toxicity No data available
2. Irritation No data available
3. Others[16] LCLo: 500mg/m3 (mouse inhalation, 10min)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[17] This substance is harmful to the environment and should be treated with special Pay attention to water pollution.
Molecular structure data
1. Molar refractive index: 14.32
2. Molar volume (cm3/mol): 51.6
3. Isotonic specific volume (90.2K ): 132.3
4. Surface tension (dyne/cm): 43.2
5. Polarizability (10-24cm3): 5.67
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 23.8
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 31.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has strong hygroscopicity and is sensitive to air and moisture. It will react violently to release hydrogen gas when it comes in contact with water.
2. Stability[18] Stable
3. Incompatible substances[19] Strong oxidants, alkalis
4. Conditions to avoid contact [20] Heat, water Vapor
5. Polymerization hazard[21] Polymerization
6. Decomposition products[22] Hydrogen bromide, hydrogen cyanide
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Storage should have suitable materials to contain spills.
Synthesis method
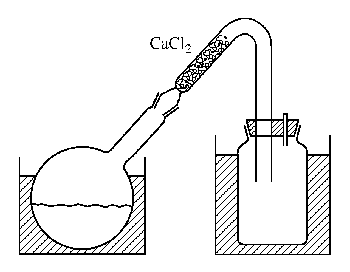
Figure XVII-17 Device for preparing cyanogen bromide
1. Picture of the device for preparing cyanogen bromide. In a well-exhausted fume hood, place 500g bromine (160mL) in an IL ground-mouth bottle cooled with ice-salt coolant. Add 50 mL of water to cover the bromine. A solution of 170g NaCN dissolved in 1200mL water was dripped in at a rate of 1 drop per second under vigorous stirring. The temperature of the reaction mixture was maintained below 20°C. Local overproduction of cyanide (CN)x should be avoided. Dilute the last 150 mL of NaCN solution with water to 2 times the original volume, and continue to add the solution, shaking the flask vigorously after each addition. Once a brown color appears that no longer disappears during shaking, the remaining NaCN solution can be discarded (it takes about 5 hours to add NaCN). Place a thick glass tube bent into a V shape on a round-bottomed flask (as shown in the picture). The shorter arm is loaded with granular CaCl2 and the CNBr is evaporated on a water bath. The product was collected in a wide-mouth bottle used as a receiver, and was in the form of snow-white crystals, 590 g, with a yield of 90% based on bromine.
2. Prepared by sodium cyanide or acrylonitrile and bromine.
Purpose
1. Cyanogen bromide is a commonly used cyanide reagent [1]. It is widely used in the field of cyanation reactions and can synthesize a large amount of cyanide. It can also be used to easily generate a variety of biologically active substances, such as urea, thiourea, selenourea, guanidine salts, hydroxyguanidine salts and a large number of other heterocyclic compounds.
Cyanogen bromide can generate electrophilic cyano groups, so electrophilic reagents such as amino groups can undergo electrophilic substitution reactions to generate cyanamide compounds. Primary amines and secondary amines react with them to obtain monosubstituted and disubstituted cyanamides (formula 1)[2] respectively.
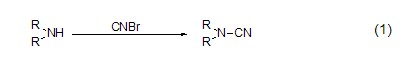
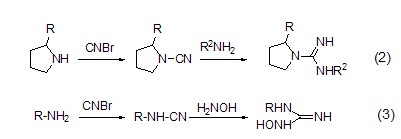
Cyanogen bromide and primary amines, After the secondary amine reacts to obtain the corresponding cyanamide compound, if it is further reacted with amine (formula 2) [3] compounds or hydroxylamine (formula 3) [4] compounds Then bioactive compounds such as guanidine salts and hydroxyguanidine salts can be synthesized.
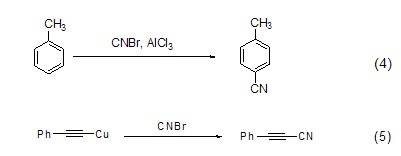
Cyanogen bromide can synthesize cyanide compounds. The reaction of toluene and cyanogen bromide under Lewis acid catalysis synthesizes aromatic cyanide compounds (formula 4)[5]; the reaction of alkynyl copper salts and cyanogen bromide produces alkynyl cyanide (formula 5)[6], this reaction provides an effective method for the synthesis of alkynyl cyanides.
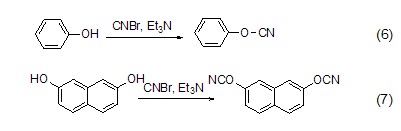
Cyanogen bromide can also synthesize cyanide compounds and dicyanoester compounds. Catalyzed by organic bases, it reacts with phenol and 2,7-naphthodiol to form cyanophenyl ester (formula 6)[7a] and 2,7-naphthodiphenol dicyanoester (formula 7) )[7b]. They are important intermediates in the field of organic synthesis and have a wide range of uses.
2. Used in organic synthesis, Alchemy, making pesticides, etc. [24]

 微信扫一扫打赏
微信扫一扫打赏

