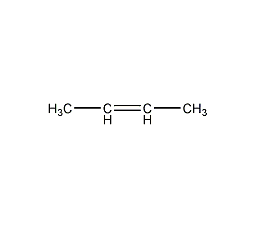
Structural formula
| Business number | 06R7 |
|---|---|
| Molecular formula | C4H8 |
| Molecular weight | 56.11 |
| label |
trans-2-butene, (E)-1,2-Dimethylethylene |
Numbering system
CAS number:624-64-6
MDL number:MFCD00064458
EINECS number:210-855-3
RTECS number:EM2932000
BRN number:1718756
PubChem number:24857756
Physical property data
1. Properties: colorless flammable gas.
2. Density (g/mL, 25/4℃): 0.6042
3. Lennard-Jones parameters (A): 9.955
4. Melting point (ºC): -140
5. Boiling point (ºC, normal pressure): 1
6. Lennard-Jones parameter (K): 145.1
7 . Refractive index (n-25D): 1.3848 span>
8. Flash point (ºC): -80
9. Solubility parameter (J·cm-3)0.5: 15.188
10. van der Waals area (cm2·mol-1): 6.400×109
11. van der Waals volume (cm3·mol-1): 44.280
12. Liquid phase standard hot melt (J·mol-1·K-1): 124.4
13. Critical temperature (K): 11.45
14. Critical pressure (MPa): 4.10
15. Critical density (g·cm-3): 0.236
16. Critical volume (cm3·mol-1): 237.7
17. Critical compression factor: 0.2735
18 . Eccentricity factor: 0.218
19. Solubility: soluble in organic solvents, insoluble in water.
20. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2727.54
21. Liquid phase standard claimed heat (enthalpy) )( kJ·mol-1): -33.0
22. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): – 2705.94
23. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -11.4
24. The gas phase standard entropy (J·mol -1·K-1): 296.33
25. Gas phase standard free energy of formation (kJ·mol-1 ): 62.9
26. Gas phase standard hot melt (J·mol-1·K-1): 87.67
Toxicological data
None yet
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1.Molar refractive index: 20.63
2. Molar volume (cm3/mol): 88.1
3. Isotonic specific volume (90.2K): 178.5
4. Surface tension (dyne/cm): 16.7
5. Dielectric constant (F/m): 2.68
6. Polarizability (10-24cm3): 8.18
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 15.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Exists in smoke and gas.
Storage method
This product should be sealed in ampoules or gas bottles and stored in a cool place.
Synthesis method
Exists in carbon four fractions obtained from refinery gas and catalytic cracking of petroleum fractions and cracking of petroleum hydrocarbons. Industrially, it is produced by the isomerization method. Using C4 raffinate as raw material, in the presence of palladium/alumina catalyst, hydroisomerization is performed to isomerize 1-butene into 2-butene, and then distilled to obtain Trans-2-butene. Derived from dehydration of butanol.
Purpose
Standard for gas chromatography analysis. Organic Synthesis. The mixture is mostly used to synthesize C4 and C5 derivatives, to prepare superimposed gasoline, cross-linking agents, etc. Mainly used for dehydrogenation to butadiene.

 微信扫一扫打赏
微信扫一扫打赏

