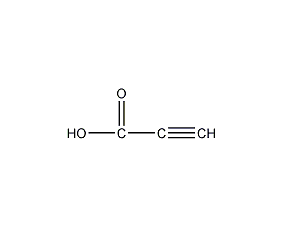
Structural formula
| Business number | 050R |
|---|---|
| Molecular formula | CHCCOOH |
| Molecular weight | 70.05 |
| label |
Acetylene carboxylic acid, Acetylenecarboxylic acid, Propynoic acid, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:471-25-0
MDL number:MFCD00004360
EINECS number:207-437-8
RTECS number:UD9300000
BRN number:878176
PubChem number:24898598
Physical property data
1. Properties: colorless liquid
2. Density (g/ m3, 25/4℃): 1.1325
3. Relative Density (20℃, 4℃): 1.1380
4. Melting point (ºC): 18
5. Boiling point (ºC, normal pressure): 74.518
6. Refractive index at room temperature (n20): 1.4306
7. Refractive index: 1.431
8. Flash point (ºF): 138
9. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1): -1273.1
10. Liquid phase Standard claimed heat (enthalpy) (kJ·mol-1): -193.3
11. Liquid phase standard entropy (J·mol-1· K-1): 226.4
12. Liquid phase standard free energy of formation (kJ·mol-1): -377.6
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
p>
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water
Toxicological data
Acute toxicity: Rat oral LD50: 100 mg/kg, no detailed instructions except lethal dose;
Rat intraperitoneal LD50: 25 mg/kg, no detailed instructions except lethal dose;
Mouse oral LD50: 100mg/kg, no detailed instructions except the lethal dose;
Small abdominal LD50: 25mg/kg, no detailed instructions except the lethal dose;
p>
Guinea pig LD50: 100mg/kg, no details except lethal dose;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 15.33
2. Molar volume (cm3/mol): 56.4
3. Isotonic specific volume (90.2K ): 152.2
4. Surface tension (dyne/cm): 52.9
5. Polarizability (10-24cm3): 6.08
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 84.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
2.It is corrosive and harmful to skin, Strong irritant to eyes and mucous membranes. Skin contact may cause burns. Gas masks and gloves should be worn. In case of eye irritation or skin contact, rinse with plenty of water.
Storage method
Seal and store at 2-8°C in a ventilated, dry place to avoid contact with other oxides. Store and transport according to general chemical regulations.
Synthesis method
1. Obtained from the oxidation of propargyl alcohol. Add propargyl alcohol to acetone and stir, add chromic anhydride sulfuric acid solution dropwise at 20°C, complete the addition, and stir at room temperature for 14 hours to generate propynoic acid.
2. React sodium acetylide and CO2 at a temperature of 3.55-7.09MPa and below 90°C to obtain it.
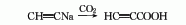
Purpose
Used in organic synthesis. It can be used to prepare propionic acid, trimesic acid, halogenated acrylic acid, dihaloacrylic acid, diacetylenedicarboxylic acid, ethyl propiolate, pyrazolone, dibromocrotonic acid, ethoxycrotonic acid, etc. Methyl propiolate (C4H4O2, [922-67-8]) can be produced by heating reflux of propionic acid with sulfuric acid and methanol, which is the raw material of the antiviral drug iodine.

 微信扫一扫打赏
微信扫一扫打赏

