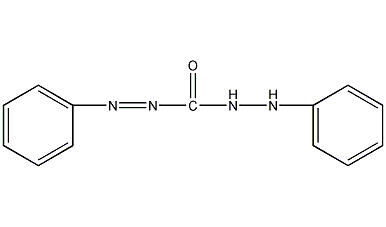
Structural formula
| Business number | 05JC |
|---|---|
| Molecular formula | C13H12N4O |
| Molecular weight | 240.26 |
| label |
Phenylazoformic acid 2-phenylhydrazide, diphenyl azocarbon hydrazide; diphenyl diamicarbazide, Reagent |
Numbering system
CAS number:538-62-5
MDL number:MFCD00003024
EINECS number:208-698-0
RTECS number:LQ9420000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: Orange-red needle-like crystals
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/ mL, air = 1): Undetermined
4. Melting point (ºC): 157
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined Determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical Temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
p>
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Dissolution Properties: Soluble in alcohol, chloroform and benzene, insoluble in water
Toxicological data
1. Acute toxicity: Rat oral LD: >500mg/kg, no details except lethal dose;
2. Mutagenicity data: DNA repairTEST system: Bacteria – Escherichia coli :1200 ng/well.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 70.42
2. Molar volume (cm3/mol): 201.0
3. Isotonic specific volume (90.2K ): 531.3
4. Surface tension (dyne/cm): 48.8
5. Polarizability (10-24cm3): 27.91
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3.6
2. Number of hydrogen bond donors: 2
3. �Number of bonded receptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecules Polar surface area (TPSA): 65.9
7, Number of heavy atoms: 18
8, Surface charge: 0
9, Complexity: 281
p>
10. The number of isotope atoms: 0
11. The number of determined atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
p>
Properties and stability
1. If stored as required, it will not decompose or react, and keep away from oxides.
2.Form red or purple complex with divalent metal ions.
Storage method
Keep sealed and placed in a ventilated, dry place.
Synthesis method
1. Obtained from the condensation of phenylhydrazine and urea, followed by oxidation with hydrogen peroxide.
2.Mix diphenylcarbohydrazide and an appropriate amount of 95% ethanol, heat to boiling, and completely dissolve it. Slowly add a small amount of potassium hydroxide while stirring, add potassium hydroxide after the reaction, filter while hot, and quickly add 3% hydrogen peroxide to the filtrate for reaction:
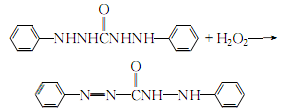
After the reaction is completed, Add 1 mol/l sulfuric acid solution under stirring until orange-red crystals precipitate, stop adding acid, and add a large amount of distilled water to dilute, filter after cooling, wash the crystals with distilled water 3 to 4 times and then dry.Diphenylcarbazone is obtained.
Purpose
1. Used as analytical reagents, chromatographic analysis reagents, adsorption indicators and complexing indicators
2.Used for photometric determination of Hg2+ , Co2+ , Cu2+ , Mn2+ and etc. are also used for indirect photometric detection of various anions coordinated with
Hg2+, such as Cl – , I–, etc., and used as indicators for precipitation titration determination of halogen, etc., in conjunction with titration determination
Hg2+, Pd2+ and other indicators. It is also used as a chromogen for the determination of barbiturates by thin layer chromatography.

 微信扫一扫打赏
微信扫一扫打赏

