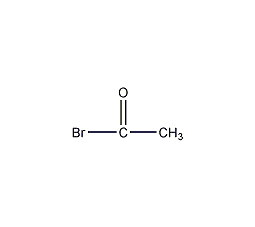
Structural formula
| Business number | 059R |
|---|---|
| Molecular formula | CH3COBr |
| Molecular weight | 122.95 |
| label |
Bromoacetyl, Acetyl bromide, Ethanoyl bromide, Dye preparations |
Numbering system
CAS number:506-96-7
MDL number:MFCD00000114
EINECS number:208-061-7
RTECS number:None
BRN number:1697546
PubChem number:24845123
Physical property data
1. Properties: colorless fuming liquid.
2. Density (g/mL, 25/4℃): 1.104
3. Relative density (20℃, 4℃): 1.662516
4. Melting point (ºC): -96
5. Boiling point (ºC, normal pressure): 76
6. Refractive index at room temperature (n20): 1.4486
7. Refractive index: 1.4576
8. Flash point (ºC): 1.67
9. Liquid phase standard Claimed heat (enthalpy) (kJ·mol-1): -223.5
10. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -190.4
11. Gas phase standard entropy (J·mol-1·K-1): 307.61
12. Gas phase standard formation free energy (kJ·mol-1): -167.0
13. Gas phase standard hot melt (J·mol-1·K-1): 70.04
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in benzene, ether and methyl chloride. It gradually turns yellow in the air and reacts violently when exposed to water or alcohol.
Toxicological data
1. Acute toxicity: Mouse intraperitoneal LC50: 250mg/kg, no details except lethal dose;
Mammalian inhalation LD50: 48 gm/m3, no details except lethal dose Description;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 19.07
2. Molar volume (cm3/mol): 71.8
3. Isotonic specific volume (90.2K ): 168.9
4. Surface tension (dyne/cm): 30.5
5. Polarizability (10-24cm3): 7.56
Computing chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 33
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications, no decomposition will occur, and avoid contact with oxides.
2.Decomposes violently when exposed to water and alcohol . This product is highly flammable. Reacts violently with water, is corrosive, and can cause burns.
3. It is toxic and highly irritating to the eyes. If used in large quantities, appropriate protective clothing, gloves and protective glasses or masks should be worn. In case of contact with eyes, rinse immediately with plenty of water and seek medical treatment. If any accident or inappropriate sensation occurs during use, please seek medical treatment. If you have any accidents or discomfort during use, please consult a doctor and keep away from fire.
Storage method
Should be kept away from fire and stored in a dry and well-ventilated place.
Synthesis method
1. Add bromine to acetic anhydride and brominate at 80°C, and then finish adding bromine between 95°C and 125°C. It takes about 2.5h. After the addition is completed, continue to reflux for 3 hours and leave overnight. Refined by fractional distillation.
2. After reacting glacial acetic acid and phosphorus trichloride at 45-50°C for 2-3 hours, reflux for 5-6 hours again,
After distillation, the crude product is obtained, and the crude product is re-distilled, and the 50-55°C fraction is taken to obtain the finished product.
Purpose
1. Organic synthesis, manufacturing dyes.
2.Used as analytical reagents. Used as acetylation reagent in organic synthesis, pharmaceutical, and dye industries.

 微信扫一扫打赏
微信扫一扫打赏

