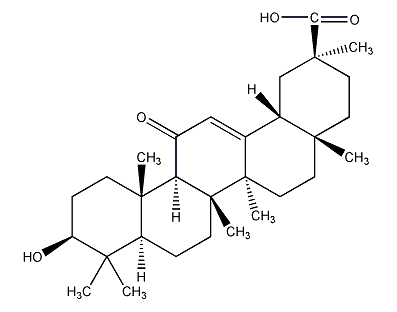
Structural formula
| Business number | 050V |
|---|---|
| Molecular formula | C30H46O4 |
| Molecular weight | 470.68 |
| label |
β-glycyrrhetinic acid; 3β-hydroxy-11-oxo-12-oleanene-30 acid, anti-inflammatories |
Numbering system
CAS number:471-53-4
MDL number:MFCD00003706
EINECS number:207-444-6
RTECS number:RK0180000
BRN number:2229654
PubChem ID:None
Physical property data
1. Appearance: White crystalline powder
2. Density (g/m3, 25/4℃): Undetermined
3 . Relative vapor density (g/cm3, air=1): Undetermined
4. Melting point (ºC): 292-295
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: 162°
8. Flash point (ºF): Undetermined
9. Specific rotation (º): 165
10. Autoignition point or ignition temperature (ºC): Undetermined
p>
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ /mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water Log value of (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V /V): Undetermined
19. Solubility: Insoluble in water, soluble in ethanol, chloroform, pyridine, and acetic acid.
Toxicological data
Acute toxicity: Mouse intraperitoneal LC50: 308mg/kg, no detailed description except the lethal dose;
Mouse intravenous LC50: 56mg/kg, no detailed description except the lethal dose;
Ecological data
Generally not hazardous to water, do not discharge material into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 133.69
2. Molar volume (cm3/mol): 411.6
3. Isotonic specific volume (90.2K ): 1082.2
4. Surface tension (dyne/cm): 47.7
5. Polarizability (10-24cm3): 52.99
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 6.4
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 5
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 34
8. Surface charge: 0
9. Complexity: 965
10. Isotopic atoms Quantity: 0
11. Determine the number of atomic stereocenters: 9
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond position Number of stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Seal and store in a ventilated, dry place to avoid contact with other oxides.
Synthesis method
1. Sweetener; sweetness improver; flavoring agent (used in conjunction with salt, the effect is particularly great); flavor enhancement Agent (for removing mutton and adding flavor to dairy products, cocoa products, egg products, mutton, etc.). Chinese folk customarily use it in sauces, sauce products, pickled products, etc. 2. Antibiotic drugs are used to treat Staphylococcus, Streptococcus, E. coli, Infections such as Helicobacter3. is an important raw material for medicine and high-end cosmetics. It has the effects of anti-inflammatory, anti-allergic, and inhibiting bacterial reproduction4. This product is an anti-inflammatory drug . Used to treat adrenal insufficiency, gastric and duodenal ulcers and other diseases. 5.Add water to the crushed licorice, heat it, extract continuously under countercurrent, separate and remove impurities, and obtain an aqueous solution. Evaporate and concentrate, add sulfuric acid while stirring to adjust the pH to 2-3. Leave to settle and remove the supernatant. The precipitate is washed with water to remove sulfate radicals, heated and concentrated to obtain glycyrrhizic acid paste. Crush the glycyrrhizic acid paste and extract it with 95% ethanol under reflux 2 to 3 times for 3 hours each time to obtain an ethanol solution. After filtration, stir and pass ammonia until the pH is 7 to 7.5. Then filter to obtain the crude triammonium glycyrrhizinate salt, which is refined with glacial acetic acid to obtain beige monoammonium glycyrrhizinate salt. Add glycyrrhizic acid monoammonium salt to 5% sulfuric acid aqueous solution, stir and heat to nearly boiling, reflux and hydrolyze for 15 hours, filter and wash with water to obtain crude glycyrrhetinic acid. After further recrystallization and decolorization, glycyrrhetinic acid is obtained.
Purpose
1. Glycyrrhizic acid extracted from the rhizome of the leguminous plant Glycyrrhiza uralen-sis fisch is made into ammonium It is obtained by hydrolysis of salt. Licorice is an annual herb in China, mainly produced in Inner Mongolia and Gansu. The roots and rhizomes of licorice mainly contain glycyrrhizin, which is the potassium and calcium salt of glycyrrhizic acid and is the sweet component of licorice. It is about 50 times sweeter than sucrose. Add water to the crushed licorice, heat it, continuously extract it under countercurrent, separate and remove impurities from the grass residue, and obtain an aqueous solution. Evaporate, concentrate, and add sulfuric acid pH 2-3 with stirring. Leave to settle and remove the supernatant. The precipitate is washed with water to remove the sulfate radicals, heated and concentrated, and boiled to make glycyrrhizic acid paste. Crush the glycyrrhizic acid paste and extract it 2-3 times with 95% ethanol for 3 hours each time to obtain an ethanol solution. After filtration, ammonia was stirred until the pH was 7-7.5. Filter again to obtain the crude triammonium glycyrrhizinate salt, which is refined with glacial acetic acid to obtain beige monoammonium glycyrrhizinate salt. Add glycyrrhizic acid monoammonium salt to 5% sulfuric acid aqueous solution, stir and heat to nearly boiling, reflux and hydrolyze for 15 hours, filter and wash with water to obtain crude glycyrrhetinic acid. After recrystallization and decolorization, base oxalic acid with a content of more than 92% is obtained. Based on licorice, the total yield is about 0.5%. 2. Made from licorice extract (see “02317 licorice”) After concentration and drying, crude crystals are obtained, which are then recrystallized in dilute alcohol. The yield is 6%-14%. It can also be extracted directly with ammonia water, concentrated and then precipitated with sulfuric acid, and then recrystallized with 95% ethanol.3. This product is an anti-inflammatory drug. Used to treat adrenal insufficiency, gastric and duodenal ulcers and other diseases. Glycyrrhetinic acid and its derivatives can be made into anti-inflammatory and anti-allergic preparations for allergic and occupational dermatitis. Adding this product to cosmetics can make therapeutic cosmetics.

 微信扫一扫打赏
微信扫一扫打赏

