Structural formula
| Business number | 04RN |
|---|---|
| Molecular formula | C3H9OBF4 |
| Molecular weight | 147.91 |
| label |
Osmium trimethyltetrafluoroborate, Trimethyloxonium tetrafluoroborate, Trimethyloxonium tetrafluoroborate, Trimethyltetrafluoroborate, Trimethyltin tetrafluoroborate, Trimethyltin Tetrafluoroborate, 97+%, Trimethyloxonium tetrafluoroborate, Trimethyl-oxoniutetrafluoroborate(1-), Trimethyloxonium tetrafluoroborate(1-), Trimethyloxonium tetrafluoroborate 98+%, Trimethyloxoniumtetrafluoroborate,C24%min, Trimethyloxoniumtetrafluoroborate,min.95%, Oxonium, trimethyl- |
Numbering system
CAS number:420-37-1
MDL number:MFCD00011798
EINECS number:206-994-4
RTECS number:None
BRN number:3597303
PubChem number:24856967
Physical property data
Character: white crystal
Density (g/mL, 25/4℃): Not available
Relative vapor density (g/mL, air=1): Not available
Melting point (ºC): 200
Boiling point (ºC, normal Pressure): Not available
Boiling point (ºC, 5.2kPa): Not available
Refractive index: Not available
Flash point (ºC): Not available
Specific rotation (º): Not available
Autoignition point or Ignition temperature (ºC): Not available
Vapor pressure (kPa, 25ºC): Not available
Saturated vapor pressure (kPa, 60ºC ): Not available
Heat of combustion (KJ/mol): Not available
Critical temperature (ºC): Not available
Critical pressure (KPa): Not available
Log value of oil-water (octanol/water) partition coefficient: Not available
Upper limit of explosion (%, V/V): Not available
Lower limit of explosion (%, V/V): Not available
Solubility: Not available
Toxicological data
2. Toxicological data:
Acute toxicity: Not available.
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. RotatableNumber of �� bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 1
7. Number of heavy atoms :9
8. Surface charge: 0
9. Complexity: 27.1
10. Number of isotope atoms: 0
11 .Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
None yet
Storage method
None yet
Synthesis method
1. Install a mechanical stirrer on the 500 ml three-neck flask, connect the T-shaped tube to the mineral oil bubble meter and the Dewar condenser of the nitrogen source, and connect the dry dimethyl ether The source of air is introduced into the tube. The bottle contains 80 ml of methylene chloride and 38.4 g (3 ml, 0.27 mol) of boron trifluoride ether solution. After a nitrogen atmosphere has been created in the bottle, the condenser is filled with a dry ice-acetone mixture. .With gentle stirring, pass dimethyl ether through the solution until approximately 75 ml has been collected. Change the gas introduction tube to a constant pressure funnel, and add 28.4 grams (24 ml, 0.307 mol) of epichlorohydrin dropwise within 15 minutes under vigorous stirring. Stir the mixture overnight under nitrogen (the dry ice in the condenser does not need to be replaced after the initial 2 to 3 hours of stirring). Replace the stirrer with a filter rod. While the mixture is still filled with nitrogen, suck out the supernatant from the mixture and use two portions of the remaining oxygen anchor salt. Wash with 100 liters of dry dichloromethane and two 100 ml portions of dry sodium ether. Then nitrogen gas is passed onto the oxygen salt to take away the ether until the smell of ether is no longer noticeable. Obtained 28-29 grams (92.5-96.5%) of self-colored crystals. 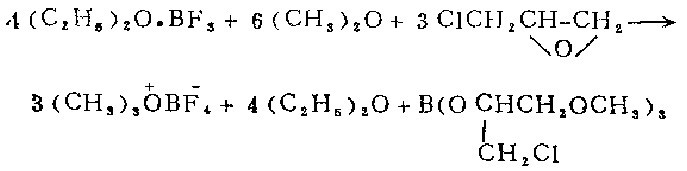
Purpose
1. Used for the oxidation of hydroxyl groups to synthesize marine natural products.
2.Strong methylating reagent. Preparation of A-acetoxyketone. Deprotection of thioacetals.

 微信扫一扫打赏
微信扫一扫打赏

