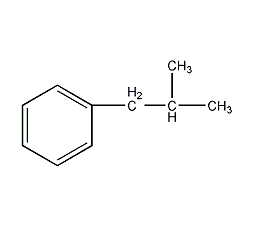
structural formula
| business number | 05jl |
|---|---|
| molecular formula | c10h14 |
| molecular weight | 134.22 |
| label |
(2-methylpropyl)benzene, 2-methyl-1-phenylpropane, (2-methylpropyl)benzene, 2-methyl-1-phenylpropane, surfactant, hydrocarbon solvents, analgesic and antipyretic ibuprofen intermediate |
numbering system
cas number:538-93-2
mdl number:mfcd00008936
einecs number:208-706-2
rtecs number:da3550000
brn number:1852218
pubchem id:none
physical property data
1. properties: colorless liquid
2. relative density: 0.8525 (20℃)
4. melting point (ºc): -52
5. boiling point (ºc, normal pressure): 173
6. boiling point (ºc, 1.60kpa): 59.5
7. refractive index: 1.4862
8 . flash point (ºc): 55
9. autoignition point or ignition temperature (ºc): 427
11. vapor pressure (kpa, 18.6ºc): 0.13
12. critical temperature (ºc): 377
13. critical pressure (kpa): 3.05
14. explosion upper limit (%, v/v): 0.8
15. lower explosion limit (%, v/v): 6.0
16. solubility: insoluble in water, soluble in ethanol and ether.
17. relative density (25℃, 4℃): 0.8491
18. refractive index at room temperature (n25): 1.4840
19. eccentricity factor: 0.381
20. solubility parameter (j·cm-3)0.5: 16.990
21. van der waals area (cm2·mol-1): 1.137×1010
22. van der waals volume (cm3·mol-1): 89.390
23. gas phase standard heat of combustion (enthalpy) (kj·mol-1): -5914.42
24. the gas phase standard claims heat (enthalpy) (kj·mol-1): -21.51
25. liquid phase standard combustion heat (enthalpy) (kj·mol-1): -5866.14
26. liquid phase standard claimed heat (enthalpy) (kj·mol-1): -69.79
27. liquid phase standard hot melt (j·mol-1·k-1 ): 251.7
toxicological data
1. acute toxicity: rat oral ldlo: 5 mg/kg, no detailed instructions except lethal dose. 2. it is harmful and irritating to the body if inhaled, taken orally or absorbed through the skin. rat experience��ld50: 2240mg/kg.
ecological data
this substance may be harmful to the environment, and special attention should be paid to water bodies.
molecular structure data
1. molar refractive index: 45.02
2. molar volume (cm3/mol): 155.6
3. isotonic specific volume (90.2k ): 360.8
4. surface tension (dyne/cm): 28.8
5. polarizability (10-24cm3): 17.84
compute chemical data
1. reference value for hydrophobic parameter calculation (xlogp): none
2. number of hydrogen bond donors: 0
3. number of hydrogen bond acceptors: 0
4. number of rotatable chemical bonds: 2
5. number of tautomers: none
6. topological molecule polar surface area 0
7. number of heavy atoms: 10
8. surface charge: 0
9. complexity: 78
10. number of isotope atoms: 0
11. determine the number of atomic stereocenters: 0
12. uncertain number of atomic stereocenters: 0
13. determine the number of chemical bond stereocenters: 0
14. number of uncertain chemical bond stereocenters: 0
15. number of covalent bond units: 1
properties and stability
1. use and store according to specifications. it will not decompose and avoid contact with oxides. it is flammable and can easily cause combustion when exposed to high heat, open flames and oxidants.
2.this product is slightly toxic, the oral dose of ld505000mg/kg in rats. this product is flammable and should be stored in a cool, ventilated warehouse, away from fire and heat sources, and stored separately from oxidants. keep the packaging intact to prevent damage.
storage method
refrigerated storage
packed in galvanized iron drum, ptfe liner, net weight 150kg.
synthesis method
using toluene and propylene as raw materials, it is obtained by carrying out side chain alkylation in the presence of an alkali metal catalyst. using metallic potassium as the catalyst, the amount of potassium used per mole of toluene is 0.02g mole (k/na2co3=4:1). the reaction temperature is 190-205°c, the pressure is 2.94mpa, the reaction time is 1.5-2.0h, and the ratio of propylene to toluene is 1:1. this method has good directionality, high yield, single product, and simple treatment process. after distillation, the pure product is obtained. the toluene conversion rate is 54.53%, the isobutylbenzene selectivity is 89.6%, and the isobutylbenzene yield is 48.88%.

purpose
used in organic synthesis. it is a raw material for the production of intermediates (isobutylacetophenone) of the analgesic and antipyretic drug ibuprofen. also used in the production of coatings, plasticizers, and surfactants. can also be used as a solvent.

 微信扫一扫打赏
微信扫一扫打赏

