Background and overview[1][2]
2-Bromo-6-nitrotoluene can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
2-Bromo-6-nitrotoluene is prepared as follows:
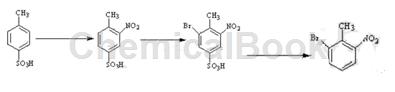
① Preparation of 3-nitro-4-methylbenzenesulfonic acid: Place 100kg p-toluenesulfonic acid and 319kg concentrated sulfuric acid into the reaction kettle, stir for 1.5 hours, and slowly add 65% concentrated nitric acid while stirring 61.8kg, keep the temperature at 30-40°C during stirring. After adding, keep stirring at 30-40°C for 1 hour to obtain the reaction mixture; then pour the reaction mixture into a mixture of 100kg ice and 200L water, and use dichloromethane to Extract with 2×500L, combine the dichloromethane layers, wash with 2×500L of water, dry the organic phase with 10kg of anhydrous magnesium sulfate, evaporate the solvent under reduced pressure to obtain 115kg of 3-nitro-4-methylbenzenesulfonic acid as a light yellow oil. Yield 92%;
② Preparation of 3-nitro-4-methyl-5-bromobenzenesulfonic acid: Add 115kg of 3-nitro-4-methylbenzenesulfonic acid in step ① to 230L of glacial acetic acid, and heat to 70°C , add 69kg liquid bromine dropwise, keep the temperature at 70°C for 12 hours, cool to room temperature, add 600L ice water, stir, filter, wash with 2×230L cold water, and dry to obtain 3-nitro-4-methyl-5-bromobenzene Sulfonic acid 130kg, yield 84%;
③ Preparation of 2-bromo-6-nitrotoluene: Add 130kg of 3-nitro-4-methyl-5-bromobenzenesulfonic acid in step ②, 39kg of sodium acetate, and 58.5kg of 40% sulfuric acid into 390L for purification In water, react at 90°C for 24 hours, cool to 5°C, suction filter, wash the filter cake fully with 2×300L cold water, and dry under reduced pressure to obtain 85.7kg of light yellow 2-bromo-6-nitrotoluene, with a yield of 90%;
Application
2-Bromo-6-nitrotoluene can be used as a pharmaceutical synthesis intermediate, such as the preparation of 2-nitro-6-trichloromethyltoluene:
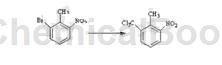
Dissolve 85.7kg 2-bromo-6-nitrotoluene in 900L dry tetrahydrofuran solution, cool to 0°C under nitrogen protection, then add 180L of 1.6M n-butyllithium hexane solution dropwise, and control the dropping speed. As long as the internal temperature does not rise more than 10°C, after the dropwise addition is completed, keep stirring at this temperature for 2.5 hours at 5°C to obtain a mixed solution;
Main reference materials
[1]CN201110432682.X A kind of synthesis method of 2-methyl-3-trifluoromethylaniline

 微信扫一扫打赏
微信扫一扫打赏

