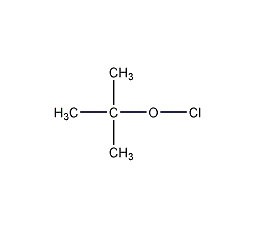
Structural formula
| Business number | 05A1 |
|---|---|
| Molecular formula | C4H9ClO |
| Molecular weight | 108.57 |
| label |
Chlorinating agent |
Numbering system
CAS number:507-40-4
MDL number:None
EINECS number:208-072-7
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Character: light yellow liquid
2. Density (g/ cm3, 25/4℃): 0.96
3. Relative Vapor density (g/cm3, air=1): Not determined
4. Melting point (ºC): Not determined
5. Boiling point (ºC , normal pressure): 78
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point ( ºF): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/ Log value of the distribution coefficient (water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
None
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 27.14
2. Molar volume (cm3/mol): 112.3
3. Isotonic specific volume (90.2K ): 244.4
4. Surface tension (dyne/cm): 22.4
5. Polarizability (10-24cm3): 10.75
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 37.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
2.Exposed to strong light, strong heat or Contact with rubber can cause it to break down violently.
Storage method
Refrigerated storage
Synthesis method
1. Note that it should be carried out in a fume hood. Strong heat and the use of rubber products are strictly prohibited.
In a 2-liter three-neck flask equipped with a stirrer, gas inlet tube and outlet tube, place 80 grams (2 mol) of sodium hydroxide and 500 ml of water. After the solid is dissolved, add a solution of 74 grams (94 ml, 1 mol) of tert-butanol and about 500 ml of water at 15-20°C and stirring at a uniform speed. First, pass chlorine gas at a rate of 1 liter/minute for 30 minutes, and then continue to pass chlorine gas at a rate of 0.5-0.6 liters/minute for 30 minutes (forward). Separate the upper organic layer and wash it several times with 50 ml of 10% sodium carbonate solution until the Congo red test paper no longer shows acidity. Wash 4 times with equal volume of water, add anhydrous calcium chloride and dry, and isolate 78-107 grams (72-99%) of the liquid product. Store in the refrigerator protected from light in the presence of inert gas.
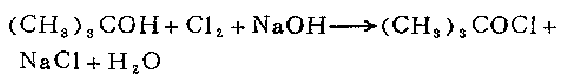
2. Attention! Operate in a dimly lit fume hood. It is strictly forbidden to contact the product with rubber. and heated to temperatures above its boiling point.
In a 1-liter Who-shaped bottle or round-bottomed flask equipped with a stirrer, add 500 ml of a solution containing 0.375-0.400 mol of sodium hypoxonite, then place it in an ice bath to cool and stir quickly until the temperature drops to Below 10℃. While protecting from light and stirring rapidly, add 37 ml (0.39 mol) tert-butanol and 24.5 ml (0.43 mol) glacial acetic acid in sequence. After continuing to stir for 3 minutes, transfer the reaction mixture to a 1-liter separatory funnel. Separate the lower water layer. The oily yellow organic layer was first washed with 50 ml of 10% sodium carbonate solution, and then washed with 50 ml of water. Add 1 gram of anhydrous calcium chloride, dry and filter to obtain a liquid product with a purity of 99-100% and a weight of 29.6-34% (70-80%). Put into dark glass bottles with calcium chloride and store in refrigerator.
3. Preparation method:
In a reaction bottle equipped with a stirrer and thermometer, heat 500mL (5% ~ 6%) sodium hypochlorite solution, cool it to below 10°C in an ice bath, and cover the reaction bottle with a black cloth to avoid strong light exposure. Stir vigorously and add a solution of 37 mL (0.39 mol) of tert-butyl alcohol (2) and 24.5 mL (0.43 mol) of glacial acetic acid one at a time, and continue stirring for 3 minutes. Pour the reaction solution into a separatory funnel, separate the aqueous layer, and wash the yellow oily organic layer with 50 mL of 10% sodium carbonate solution and 50 mL of water, dry over anhydrous calcium chloride, and filter to obtain tert-butyl hypochlorite (1 ) 30~34g, yield 70%~80%, purity 99%~100%. Store in a brown bottle with calcium chloride desiccant in the refrigerator. [3]
4. In a reaction bottle equipped with a stirrer and ventilation tube, add 50mL water and 80g sodium hydroxide (2.0mol), stir and dissolve, and then cool Until chlorine gas is introduced for 30 minutes, then chlorine gas is introduced at a rate of 0.5 to 0.6L per minute for 30 minutes. Pour the reaction solution into a separatory funnel, separate the aqueous layer, and wash the yellow oily organic layer with 50 mL of 10% sodium carbonate solution each time until it is no longer acidic to Congo red, and then wash it four times with water and anhydrous calcium chloride. Dry and filter to obtain tert-butyl hypochlorite ① (1) 78 to 107 g, with a yield of 72% to 99%. Store in a refrigerator with calcium chloride desiccant and inert gas protected brown bottles. Note: ① The product contains about 2% free chlorine, which is pure enough for most uses. It can be purified by distillation, bp77~78℃. Through the measurement of active chlorine, the purity before purification is 97% to 98%, and the purity after purification is 98% to 100%. [4]
Purpose
Effective chlorinating agent. Used for the chlorination of hydroxyl groups (such as the preparation of allyl chloride), the chlorination of ketones, the N-chlorination of certain nitrogen-containing compounds and the photochlorination of ethers, etc. oxidizing agent. Oxidize alcohol to form ketone (aldehyde) or a chloroketone, and oxidize thioether to form sulfoxide.

 微信扫一扫打赏
微信扫一扫打赏

