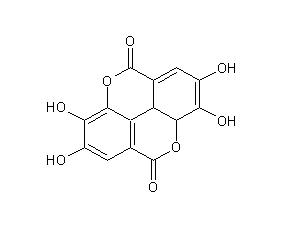
Structural formula
| Business number | 051C |
|---|---|
| Molecular formula | C14H6O8 |
| Molecular weight | 302.19 |
| label |
2,3,7,8-tetrahydroxy(1)benzopyrano(5,4,3-cde)(1)benzopyran-5,10-dione, 4,4′,5,5′,6,6′-Hexahydroxydiphenic acid 2,6,2′,6′-dilactone, Digallolactone;, food antioxidants |
Numbering system
CAS number:476-66-4
MDL number:MFCD00149494
EINECS number:207-508-3
RTECS number:DJ2620000
BRN number:7549
PubChem ID:None
Physical property data
1. Character: milky white crystal
2. Density (g/ m3, 25/4℃): 1.667
3. Melting point ( ºC): 35
4. Solubility: Slightly soluble in water and ethanol. Soluble in alkaline aqueous solution and organic solvents such as benzene and ethyl acetate
Toxicological data
Reproductive Toxicity: Mouse DOSE Subcutaneous TDLo: Female after 16 days of pregnancy: 1208 mg/kg SEX/DURATION, abortion, specific developmental abnormalities – blood and lymphatic system (including spleen, bone marrow)
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 66.59
2. Molar volume (cm3/mol): 146.8
3. Isotonic specific volume (90.2K ): 507.5
4. Surface tension (dyne/cm): 142.6
5. Polarizability (10-24cm3): 26.39
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.1
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 8
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 127
6. Topological molecular polar surface area 134
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 475
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications. It will not decompose and avoid contact with oxides.
Storage method
Seal and store in a ventilated, dry place to avoid contact with other oxides.
Synthesis method
Mainly extracted from plants, it is abundant in gallnuts produced by Javanese sumac, water chestnut fruits, terminalia fruits, and eucalyptus globulus leaves. The raw materials are usually defatted. and then leached with alkaline aqueous solution orPure ellagic acid can be obtained by leaching with ethanol, then removing water-soluble proteins and gliadins, and then using enzymes to decompose and remove sugar ligands.
Purpose
1. Used in medicine and cosmetics as an antioxidant, with anti-cancer and virus-inhibiting effects.
2.Ellagic acid has anti-cancer/anti-mutation properties, has an inhibitory effect on human immunodeficiency virus, and is effective against a variety of bacteria and viruses. Inhibitory effect, antihypertensive and sedative effects.

 微信扫一扫打赏
微信扫一扫打赏

