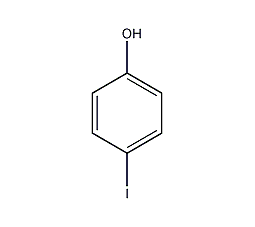
Structural formula
| Business number | 05K6 |
|---|---|
| Molecular formula | C6H5OI |
| Molecular weight | 220.01 |
| label |
4-iodophenol, p-iodophenol, 4-iodophenol, 4-Iodophenol, 4-Hydroxyiodobenzene |
Numbering system
CAS number:540-38-5
MDL number:MFCD00002327
EINECS number:208-745-5
RTECS number:SL5600000
BRN number:1904544
PubChem ID:None
Physical property data
1. Characteristics: colorless needle-shaped crystals
2. Density (g/ cm3,25/4℃): Undetermined
3. Relative vapor density (g/cm3,air=1): Undetermined
4. Melting point (ºC):92-94
5. Boiling point (ºC,Normal pressure):138
6. Boiling point (ºC,8kPa): Undetermined
7. Refraction Rate: Undetermined
8. Flash point (ºC):138
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure (kPa,55.1ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of partition coefficient (water): undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility:Slightly soluble in water, easily soluble in organic solvents such as ethanol and ether
Toxicological data
1 , acute toxicity: mice through abdominal cavityLDLo: 700mg/kg, no details except lethal dose;
2 , tumorigenic data: mice through skinTDLo: 7200 mg/kg/18W-I, RTECS Standard Skin & Attachment–Tumor;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index:41.04
2. Molar volume (m3/mol):109.9
3. Isotonic specific volume (90.2K): 297.8
4. Surface tension (dyne/cm): 53.9
5. Polarizability(10-24cm 3):16.27
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 66.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Use and store according to specifications, no decomposition will occur, and avoid contact with oxides
Storage method
Stored in a cool, dry, well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is obtained by diazotization and replacement of p-aminophenol. The mixture of p-aminophenol, water and sulfuric acid was cooled to 0°C with ice cooling, and the sodium nitrite solution was added within 1 hour while stirring. After the addition is completed, continue stirring for 20 minutes, then add concentrated sulfuric acid to obtain a diazotization liquid. Pour the diazotization solution into the ice-cooled potassium iodide solution, then add copper powder, and stir at 75-80°C until no nitrogen is released. Cool to room temperature, extract with chloroform three times, combine the extracts, and wash with dilute sodium thiosulfate solution. Distillation to recover chloroform. The residue is distilled under reduced pressure, and the 135-140°C (61.3kPa) fraction is collected and cooled for crystallization to obtain crude p-iodophenol. Crystallize with dilute ethanol to obtain fine product. The yield is about 70%.
Purpose
This product is condensed with chloroacetic acid to obtain p-iodophenoxyacetic acid. This is a plant growth regulator, also known as Yield-increasing Spirit; it is also used to fatten pigs, so it is also called Fat Pig Spirit. Iodophenol is also used in other organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

