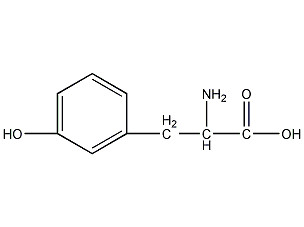
Structural formula
| Business number | 05Z2 |
|---|---|
| Molecular formula | C9H11NO3 |
| Molecular weight | 181.19 |
| label |
L-M-tyrosine,98%, L-M-tyrosine, L-Metatyrosine, 3-HYDROXY-L-PHENYLALANINE, H-S-META-TYR-OH, H-M-TYR-OH, L-3-HYDROXY-PHENYLALANINE, L-M-TYR, L-M-TYROSINE, L-META-TYROSINE, META-TYROSINE, Phenylalanine analogs alpha and other aromatic amino acids, Amino acids and derivatives |
Numbering system
CAS number:587-33-7
MDL number:MFCD00063059
EINECS number:None
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Physical property data
1. Properties: colorless or light yellow transparent liquid.
2. Melting point17-18℃,
3. Boiling point213-214℃,55℃(0.133kPa),
4. Relative density1.2410(20/4℃ ) ,
5.Refractive index1.5650.
6. Soluble in ethanol, ether, benzene and acetone, slightly soluble in water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
5. Molecular property data: 1、 Molar refractive index:47.37 2、 Molar volume (m3/mol):135.8 3、 Isotonic specific volume (90.2K):386.8 4、 Surface tension (dyne/cm): 65.7 5, Polarizability (10-24cm3 ):18.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 9
6. Topological molecule polar surface area 83.6
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 184
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
In living organisms it May be a precursor or intermediate of catecholamines. Para-hydroxylation of phenylalanine in vivo by meta-hydroxylase(For example, meta-hydroxylation is carried out before the formation of dopa, followed by decarboxylation to generate dopamine,dopamine). L-m-Tyrosine can penetrate the blood-brain barrier and decarboxylate into tyrosinem-Tyramine, which has the effect of stimulating dopamine receptors.
Storage method
None yet
Synthesis method
2. Introduction to production methods
It is available with Euphorbia Family, Euphorbia(Euphorbia myrsztiites L.), it can also be obtained by semi-synthetic method.
Purpose
3. Purpose
Currently it is mainly used in biochemical reagents.

 微信扫一扫打赏
微信扫一扫打赏

