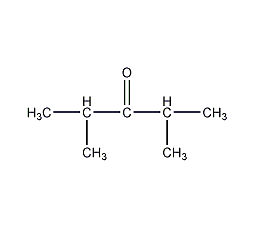
Structural formula
| Business number | 05TP |
|---|---|
| Molecular formula | C7H14O |
| Molecular weight | 114.19 |
| label |
diisopropyl ketone, Diisopropyl ketone, Isobutyrone, Isopropyl ketone, Rare and precious metal extractants |
Numbering system
CAS number:565-80-0
MDL number:MFCD00008918
EINECS number:209-294-7
RTECS number:SA8575500
BRN number:773782
PubChem number:24848297
Physical property data
1. Properties: colorless flammable liquid.
2. Boiling point (ºC, 101.3kPa): 124.4
3. Melting point (ºC): -69.0
4. Relative density (g/mL, 20/4ºC): 0.8108
5. Refractive index (20ºC): 1.400
6. Flash point (ºC): 15
7. Solubility: Miscible with ethanol and ether, slightly soluble in water, soluble in benzene.
8. Relative density (25℃, 4℃): 0.7986
9. Refractive index at room temperature (n20): 1.3999
10. Refractive index at room temperature (n25): 1.3976
11. Solubility parameter (J·cm-3)0.5 : 17.881
12. van der Waals area (cm2·mol-1): 1.122×1010
13. van der Waals volume (cm3·mol-1): 79.940
14. Gas phase Standard heat of combustion (enthalpy) (kJ·mol-1): -4454.04
15. Gas phase standard claims heat (enthalpy) (kJ·mol-1 sup>): -311.33
16. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -4402.49
17. Liquid Phase standard claims heat (enthalpy) (kJ·mol-1): -352.88
18. Liquid phase standard hot melt (J·mol-1·K-1):236.7
Toxicological data
1. Acute toxicity: LD50: 3536 mg/kg (rat oral); LD50: >16120 mg/kg (rat transdermal)
LD50: >2765ppm, 6 hours (large Rat inhalation)
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 34.42
2. Molar volume (cm3/mol): 141.9
3. Isotonic specific volume (90.2K): 310.4
4. Surface tension (dyne/cm): 22.8
5. Polarizability (10– 24cm3): 13.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 72.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid breathing vapors. It is easy to cause combustion when exposed to high heat, open flames and strong oxidants.
2. Exist in smoke.
Storage method
Seal the secret container and store it in a sealed main container in a cool, dry place.
Synthesis method
1. Preparation method:
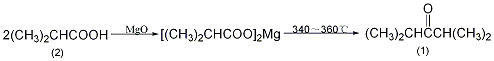
Add 90g (1mol) of isobutyric acid (2) and 90mL of water into the beaker. Slowly add 21g of magnesium oxide while stirring. Stir thoroughly. The reaction will be exothermic. Evaporate to dryness and grind into powder. In a reaction flask equipped with a distillation device and a thermometer (extending close to the bottom of the reaction flask), add 250g (about 2.4 mol) of the magnesium salt of isobutyric acid mentioned above. Slowly heat to 300°C over low heat, and liquid will slowly distill out. Raise the temperature to 340~360℃ and maintain this temperature until there is no distillate in the reaction bottle. The distillate is saturated with calcium chloride, the organic layer is separated, washed with 10% sodium carbonate and saturated brine in sequence, dried over anhydrous magnesium sulfate, fractionated, and the fractions between 121 and 125°C are collected to obtain diisopropyl ketone (1) 99g, yield 41%. [1]
Purpose
Used as rare precious metal extraction agent and solvent.

 微信扫一扫打赏
微信扫一扫打赏

