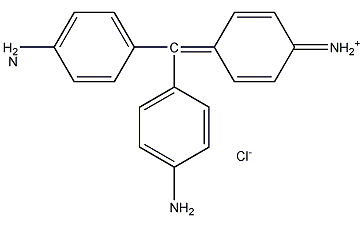
Structural formula
| Business number | 05TU |
|---|---|
| Molecular formula | C19H17N3 · HCl |
| Molecular weight | 323.82 |
| label |
Basic parafuchsin, Paramagenta hydrochloride, Pararosaniline chloride, Pararosaniline hydrochloride, Parafuchsin hydrochloride;, biological stains |
Numbering system
CAS number:569-61-9
MDL number:MFCD00001657
EINECS number:209-321-2
RTECS number:CX9850100
BRN number:4164603
PubChem number:24888442
Physical property data
1. Physical property data
1. Properties: Green shiny crystals or brown-red powder.
2. Melting point (℃): 268-270
3. Solubility: Easily soluble in ethanol, crimson, hot water, slightly soluble in cold water, insoluble in ether .
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 75.9
7. Number of heavy atoms: 23
8. Surface charge: 0
9. Complexity: 457
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Stable under normal temperature and pressure.
Storage method
Sealed and dry storage
Synthesis method
1. Add aniline, diaminodiphenylmethane, aniline hydrochloride, nitrobenzene and powdered ferric chloride to the reactor in a certain proportion and heat while stirring to 100°C, mix it into a uniform oil, continue to heat to 150°C, and maintain reflux. At this time, water will evaporate. When the water no longer evaporates, stop heating and add hot water, and then slowly add hydroxide while it is hot. Sodium solution until the red color of the water layer disappears, steam distillation to evaporate aniline, add 30% hydrochloric acid to the alkaline raffinate of phenolphthalein until it becomes slightly acidic, and heat to boiling. Stop heating, add sodium carbonate solution to the filtered filtrate until the red color of the filtrate disappears, and parafuchsin is precipitated. Filter out parafuchsin, wash with water, press dry, dissolve with ethanol, and add concentrated sulfuric acid to The red intensity no longer increases, then heat to boiling, filter while hot, and cool the filtrate.The precipitated product is pure parafuchsin.
Purpose
1. Biological staining
2. Used as a chromogenic reagent for spectrophotometric determination of sulfur dioxide content in the atmosphere. Also used as biological stain.

 微信扫一扫打赏
微信扫一扫打赏

