Overview[1][2]
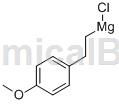
Grenya reagent, named after its discoverer V.A. Grginard, is very reactive and easily reacts with water, carbon dioxide, alcohols, aldehydes, ketones, esters, amines and epoxy compounds to generate various types of organic compounds. It is an important reagent in organic synthetic chemistry and is also widely used in the synthesis of elemental organic compounds. 4-Methoxystyryl magnesium chloride is a kind of Grenja reagent.
Apply[1-2]
4-Methoxystyrylmagnesium chloride can be used to prepare N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-2-furan-2-yl-2- Hydroxy-4-(4-methoxyphenyl)butanamide: To 2 g (0.014 mol) of 2-furan-2-yl-2-oxoacetic acid and two drops of DMF in 40 ml CHCl3 (without ethanol) cooled To the suspension at 0°C, add 1.17ml (0.013mol) oxalyl chloride. After the resulting mixture was warmed to room temperature with stirring, stirring was continued for 1 hour. The mixture was then concentrated to dryness in vacuo, and the resulting residue was dissolved in CHCl3 (20 ml) and concentrated again. Repeat this step twice. The product obtained was dissolved in CHCl3 (50 ml), the solution was cooled to 0°C, and 1.7 g (0.013 mol) (3R)-aminoquinuclidine was added. The mixture was allowed to warm to room temperature and stirring was continued for 16 hours. The reaction mixture was then treated with K2CO3 (saturated solution) and the aqueous layer was extracted with CH2Cl2. The organic layers were combined, washed with water, dried over Na2SO4, filtered and evaporated. The obtained product (N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-2-(2-furyl)-2-oxoacetamide) was used without further purification in the following Describe the steps.
The product was dissolved in anhydrous THF (50 ml). After the resulting solution was cooled to -80°C, 4-methoxystyryl magnesium chloride (0.021 mol, 43 ml of 0.5 M THF solution) was added as a Grignard reagent. The mixture was allowed to warm to room temperature for 3 hours. The reaction mixture was then treated with NH4Cl (saturated solution) and extracted with AcOEt and CH2Cl2. The organic layers were combined, dried over Na2SO4, filtered and the solvent was evaporated. The obtained product was purified by column chromatography (silica gel, CHCl3:MeOH:NH4OH99:1:1→CHCl3:MeOH:NH4OH97:3:1 as eluent) to obtain 2.4g of the title compound (44% of the starting material acid). It is a mixture of diastereoisomers.
Main reference materials
[1]Dictionary of Chemical Substances
[2]CN03820648.X Quinuclidine derivatives

 微信扫一扫打赏
微信扫一扫打赏

