Background and overview[1]
1,2-bis(4-bromobenzene)-1,2-stilbene can be used to prepare a class of processable organic small molecule materials with excellent properties.
Preparation[1]

Place 4-bromo-benzophenone, Zn powder, and dry tetrahydrofuran in a double-necked round-bottomed flask. Degas after the temperature is constant in a low-temperature reactor at -78°C. Add TiCl4 dropwise into the round-bottomed flask. In the bottom flask, stir for 20 minutes, remove the low-temperature reactor, and continue stirring at room temperature for 30 minutes. Then move to an 80°C oil bath and reflux and stir for 24 hours. After the reaction is completed, cool to room temperature, and add a small amount of water to quench the reaction. Extract with dichloromethane, concentrate the extract, and separate by column chromatography (eluent: petroleum ether: dichloromethane 6:1) to obtain a white solid 1,2-bis(4-bromophenyl)-1,2 -Diphenylethylene.
Apply[1]
It can be used to prepare the following end products:
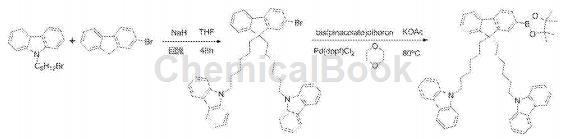
Dissolve 2-bromofluorene in dry tetrahydrofuran, add it dropwise to the sodium hydride that has removed the oxide film, activate for 1 hour, dissolve N-(6-bromo-hexane)-carbazole in dry tetrahydrofuran in, and added to the system drop by drop, refluxed for 8 hours, cooled to room temperature after the reaction, filtered the excess sodium hydride, concentrated the filtrate, and separated by column chromatography (eluent: petroleum ether: dichloromethane 4:1), 2-Bromo-9,9-(N-carbazole-hexyl)fluorene was obtained as a white solid.
Dissolve 2-bromo-9,9-(N-carbazole-hexyl)fluorene, pinacol diborate and anhydrous potassium acetate in dioxane, and add the catalyst Pd(dppf )Cl2, the reaction system was refluxed and stirred at 80°C for 48 hours. After the reaction was completed, it was cooled to room temperature, an appropriate amount of water was added, extracted with dichloromethane, the organic phases were combined, and the extract was concentrated. Chromatographic separation (eluent is petroleum ether: dichloromethane 3.5:1) to obtain a white solid 2-(4,4,5,5-tetramethyl-1,3,2dioxaborane).�)-9,9-(N-carbazole-hexyl)fluorene.
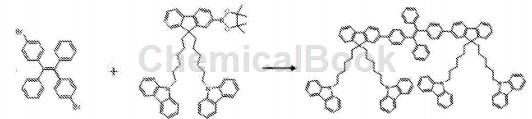
Place 1,2-bis(4-bromophenyl)-1,2-diphenylethylene and 2-(4,4,5,5-tetramethyl-1,3,2-dioxa Pentaborane)-9,9-(N-carbazole-hexyl)fluorene, chromatographically pure toluene, sodium carbonate aqueous solution (2M/L), and absolute ethanol were placed in a round-bottomed flask, and the catalyst Pd ( PPh3)4, the reaction system was refluxed and stirred at 90°C for 48 hours. After the reaction was completed, the system was cooled to room temperature, an appropriate amount of water was added to the system and then dichlorinated with After extraction with methane, the extract was concentrated and separated by column chromatography (eluent was petroleum ether: dichloromethane 4:1) to obtain the target product as a light yellow solid.
A matrix-assisted time-of-flight mass spectrometer was used to perform mass spectrometry detection on the obtained product. The obtained mass spectrum m/e (number of protons/number of charges, that is, mass-to-charge ratio): 1659.9. The obtained product was subjected to NMR detection, and the obtained NMR spectrum analysis data are as follows: 1HNMR (500MHz, CDCl3) δ8.09 (d, J=7.7Hz, 1H),8.06(d,J=7.7Hz,1H),7.71(dd,J=7.6, 4.7Hz,1H),7.64(dd,J=11.2,7.8Hz,1H),7.55(d,J=7.9 Hz,1H),7.49(t,J=9.0Hz,1H), 7.47–7.43(m,1H),7.43(s,1H),7.40(d,J=5.6Hz,1H),7.38(d,J =7.9Hz,1H),7.31(d,J=8.2Hz,1H),7.27(t,J=3.3Hz,1H),7.24(d,J=4.9Hz,1H),7.23(s,1H), 7.22(d,J=2.8Hz, 1H),7.20(s,1H),7.18(d,J=6.9Hz,2H),7.15(d,J=8.2Hz,1H),7.14–7.11(m,1H ), 4.17 (t, J=7.2Hz, 1H), 4.10 (t, J=7.2Hz, 1H), 1.91 (dt, J=22.0, 8.2Hz, 2H), 1.69 (dt, J= 14.8, 7.4Hz ,1H),1.62(dd,J=14.4,7.1Hz,1H),1.21–0.98(m,4H),0.60(dt,J=21.6, 7.3Hz,2H).
Main reference materials
[1] CN201710503006.4 A compound, luminescent material, organic light-emitting device and display device

 微信扫一扫打赏
微信扫一扫打赏

