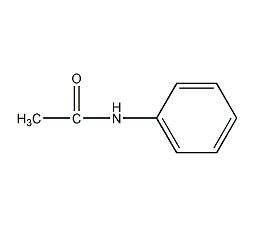
Structural formula
| Business number | 05ZF |
|---|---|
| Molecular formula | C9H11NO |
| Molecular weight | 149.19 |
| label |
N-benzylacetamide, N-acetyl benzylamine, N-Benzyl acetamide, N-acetyl benzylamine, N-benzylacetamide, BENZYLACETAMIDE, N-ACETYLBENZYLAMINE, N-BENZYLACETAMIDE, Acetamide, N-benzyl-, Acetamide,N-(phenylmethyl)-, Benzylpiperazine-M (benzylamine), AC, n-(phenylmethyl)-acetamid, n-(phenylmethyl)acetamide |
Numbering system
CAS number:588-46-5
MDL number:MFCD00059204
EINECS number:209-619-2
RTECS number:AB4546300
BRN number:None
PubChem ID:None
Physical property data
1. Physical property data
1. Boiling point 157°C / 2mmHg
2. Melting point (℃): 61
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
Molecular property data:
1, Molar refractive index:44.05
2, Molar volume (m3/mol):144.6
3, Isotonic specific volume (90.2K):356.6
4, Surface tension (dyne/cm):36.9
5, Polarizability (10-24cm3 ):17.46
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 29.1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 128
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
1. Introduction to production methods
Use benzyl chloride to Amination of ammonia water produces benzylamine hydrochloride, which is neutralized with alkali to obtain benzylamine, which is then acylated with acetic acid to obtain this product.
Purpose
2. Purpose
Sulfamirone vinegar Intermediates of acid salts.

 微信扫一扫打赏
微信扫一扫打赏

