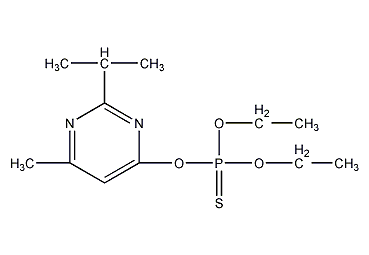
Structural formula
| Business number | 049L |
|---|---|
| Molecular formula | C12H21N2O3PS |
| Molecular weight | 304.35 |
| label |
O,O-diethyl-O-(2-isopropyl-4-methylpyrimidinyl)phosphorothioate, Thianon, diazinon, Dimpylate, Bazudin, Ciazinon, Dassitox, Dimpylat, Neocidol, Spectracide, O,O-Diethyl-O-(2-isopropyl-4-methylpyrimidyl)thiophosphate, Organophosphorus pesticides |
Numbering system
CAS number:333-41-5
MDL number:MFCD00036204
EINECS number:206-373-8
RTECS number:TF3325000
BRN number:273790
PubChem number:24868562
Physical property data
1. Physical property data
Character: colorless oil
Density (g/mL, 25/ 4℃): 1.116-1.118
Relative vapor density (g/mL, air=1): Not available
Melting point (ºC) : Not available
Boiling point (ºC, normal pressure): 83-84
Boiling point (ºC, 5.2kPa): Not available
p>
Refractive index: 1.498
Flash point (ºC): Not available
Specific rotation ( º): Not available
Autoignition point or ignition temperature (ºC): Not available
Vapor pressure (kPa, 25ºC): 18700
Saturated vapor pressure (kPa, 60ºC): Not available
Heat of combustion (KJ/mol): Not available
Critical temperature (ºC): Not available
Critical pressure (KPa): Not available
Oil and water (Xin Log value of the partition coefficient (alcohol/water): not available
Upper explosion limit (%, V/V): not available
Lower explosion limit (%, V/V): Not available
Solubility: At room temperature, the solubility in water is 40mg/L (40ppm), miscible with ethanol, acetone, and xylene. Soluble in glycerol
Toxicological data
2. Toxicology data:
Acute toxicity: LD50: 76 mg/kg (orally in rats); 85 mg/kg (orally in mice) mouth) .
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 79.64
2. Molar volume (cm3/mol): 260.4
3. Isotonic specific volume (90.2K): 677.2
4. Surface tension (dyne/cm): 45.6
5. Polarizability (10-24cm 3): 31.57
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 7
5. Number of tautomers: 3
6. Topological molecule polar surface area 85.6
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 307
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It is mildly irritating to the skin and eyes of rabbits. At the test dose, it has no teratogenic, carcinogenic or mutagenic effects on animals.
Storage method
None yet
Synthesis method
1. React methanol, hydrogen chloride and isobutyronitrile to generate the corresponding imide ester hydrochloride; treat it with ammonia and convert it into the corresponding amidine. Then, under alkaline conditions, the amidine is condensed with ethyl acetoacetate to obtain pyrimidine alcohol (4-hydroxy-2-isopropyl-6-methylpyrimidine). Pyrimidinol is heated to reflux in the presence of sodium carbonate in benzene or toluene, diethyl thiophosphoryl chloride and copper nitrate are added, and the mixture is condensed to obtain diazinon.
2.React methanol, hydrogen chloride and isobutyronitrile to generate the corresponding imide ester hydrochloride; treat it with ammonia to obtain isobutylamidine, Under alkaline conditions, isobutylamidine is condensed with ethyl acetoacetate to obtain pyrimidine alcohol (4-hydroxy-2-isopropyl-6-methylpyrimidine). Pyrimidine alcohol is heated to reflux in the presence of sodium carbonate in benzene or toluene, diethyl thiophosphoryl chloride and copper nitrate are added, and the mixture is condensed to obtain diazinon.
3.For the preparation of 0,O-diethylphosphorothioate chloride, please refer to the synthesis of phosphos. Preparation of 2-isopropyl-4-methyl-6-hydroxypyrimidine from isobutyl Nitrile reacts with acetonitrile and hydrochloric acid to produce amine ether hydrochloride, which is then reacted with helium to produce isobutyric acid. 2-Isopropyl-4-methyl-6-hydroxypyrimidine is prepared by cyclization of isobutyl ethyl acetate with diethyl ether acetate in the presence of sodium hydroxide.
4.The synthesis of diphosphine will be 2-isopropyl-4-methyl After mixing 6-hydroxypyrimidine, 0.638 SMB, 2.828 sodium hydroxide and SOm-butanone, stir 0.Sh at room temperature, slowly raise the temperature to 55 (‘, until the material is clear, add 9.5} O, O-2 Ethyl phosphorothioate K4} chloride, and raise the temperature to 70°C, stir for 0.5 hours, reflux for 1 hour, stir to cool, add SOm water, dissolve the precipitated solid, and adjust the pH value with 20°C NaOH to 11, stir and layer. Extract the water layer with methyl ethyl ketone, combine the ethyl alcohol, and remove the ethyl alcohol under reduced pressure. After the residue is washed with alkali, pickled, and then alkaline, washed until neutral, heated and dehydrated under reduced pressure to obtain 14g. Brown transparent liquidLiquid, refractive index 1.49420
Purpose
1. It is a non-systemic insecticide and has certain acaricidal effect. Mainly used for leaf-eating and sucking mouthparts pests on rice, fruit trees, grapes, sugar cane, corn, tobacco and horticultural plants
2. It is a broad-spectrum organophosphorus insecticide with contact killing, gastric poisoning, fumigation effects, and good acaricidal and ovicide effects. Used to control apple beetles with the same effect as parathion. Controls grubs or wireworms more effectively than parathion. In addition to being used as a general contact insecticide, it can also be injected into cattle to kill the larvae of the cattle tumor fly. Less toxic to livestock. It can be processed into wettable powder, emulsion and powder. In addition to copper-containing fungicides and alkaline pesticides, it can be mixed with most pesticides.

 微信扫一扫打赏
微信扫一扫打赏

