Adipic acid, also known as fat acid, is a carboxylic acid organic compound. It is a white crystalline powder, slightly soluble in water, and is acidic when dissolved in water. As the basic building block of a variety of chemical products, adipic acid is by far the most important aliphatic dicarboxylic acid in industrial production. Globally, approximately 3 million tons of adipic acid are used to synthesize nylon-66 monomers every year. In addition, adipic acid is also used to synthesize polyester, polyurethane, lubricant, plasticizer, adiponitrile, and gelling aid used as seasoning in food.

Preparation
The conventional preparation method of adipic acid is completed in two steps: first, in the presence of catalyst cobalt or catalyst cobalt and anhydrous metaboric acid, cyclohexane is oxidized by air into cyclohexanol and cyclohexanone (KA oil) mixture, and then KA oil is oxidized to obtain adipic acid. This process has been mature as early as the 1940s. It involves oxidation of KA oil with 40-60% nitric acid under the catalysis of copper and vanadium, and then eliminates nitrogen and oxygen. After the compound is mixed with water, it is crystallized in nitric acid to obtain adipic acid. The by-products glutaric acid, succinic acid, valeric acid and caproic acid produced in this process are further refined to obtain adipic acid. The gas by-products generated in this process include carbon oxides and nitrogen oxides. Among them, NO and NO2 can be completely recovered and returned to the process production in the form of nitric acid, while N2O cannot be recovered and must undergo downstream treatment.
Although manufacturers of adipic acid have adopted N2O emission reduction technologies to reduce N2O emissions since 1996, the production of adipic acid is still the main industrial source of N2O emissions. In 1998, Sato, Aoki and Noyori reported a method of directly oxidizing cyclohexene to adipic acid using hydrogen peroxide aqueous solution under organic solvent-free conditions (Figure 1). This method uses Na2WO4˙2H2O as a catalyst, [ CH3(n-C8H17)3N]HSO4 (PTC) is used as a phase transfer catalyst, using 1 mol% catalyst, 1 mol% PTC and 4.4 equiv (10% excess) of 30% H2O2 aqueous solution. Cyclohexene is heated at 90°C. The conversion to adipic acid can be completed within 8 hours.
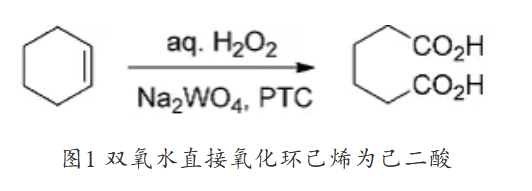
Figure 1 Direct oxidation of cyclohexene to adipic acid with hydrogen peroxide
However, the oxidation route from hydrogen peroxide to directly oxidizing cyclohexene to adipic acid has certain obstacles in industrial application. Ammonium bisulfate PTC needs to achieve full contact between the hydrophobic matrix, hydrophilic reagents and catalysts ( Peroxytungstate is soluble in water but immiscible with cyclohexene), however, this demonstrates that PTC can be replaced by organic ligands coordinated on the tungstate catalyst. In addition, it has been reported that adipic acid can also be prepared from cyclohexene and H2O2 aqueous solution in the absence of any PTC. Recently, this conversion was carried out at pilot scale in continuously stirred tank reactors (CSTRs) using tungstic acid (H2WO4) as catalyst and H2SO4 and H3PO4 as cocatalysts. Unfortunately, the preparation of adipic acid requires a reaction at 90°C for 10 hours, thus requiring a fairly large reactor to achieve industrially relevant productivity.
Purpose
Adipic acid is the most valuable dibasic acid among aliphatic dibasic acids. Adipic acid has the general properties of aliphatic dibasic acids, including salt-forming reactions, esterification reactions, amidation reactions, etc. The main use of adipic acid is as a raw material for three major categories of products, namely: the synthesis of nylon 66 salt, and then the manufacture of polyamide resin and fiber (nylon 66). Nylon 66 is the largest end market for adipic acid; the synthesis of polyester polyols , used in the production of polyester polyurethane; adipate diester plasticizer. In addition, it can also be used to produce high-grade lubricants and food additives (sour agents for food and beverages).
Polyurethane is an important application field of adipic acid. Adipic acid-based polyester diols (polyols) are used in the production of polyurethane elastomers, polyurethane leather, polyurethane shoe materials, polyurethane adhesives, etc.

 微信扫一扫打赏
微信扫一扫打赏

