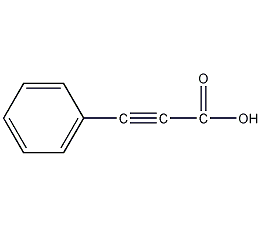
Structural formula
| Business number | 070P |
|---|---|
| Molecular formula | C9H6O2 |
| Molecular weight | 146.14 |
| label |
phenylpropinoic acid, Phenylacetylenecarboxylic acid, Phenylpropynoic acid, C6H5C≡CCOOH, Alkynoic acid derivatives |
Numbering system
CAS number:637-44-5
MDL number:MFCD00004361
EINECS number:211-285-8
RTECS number:None
BRN number:742587
PubChem number:24887398
Physical property data
1. Properties: White lens powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 135-137
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Undetermined.
Toxicological data
None
Ecological data
Do not allow undiluted or large quantities of products that are slightly hazardous to water to come into contact with groundwater, waterways or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 40.30
2. Molar volume (cm3/mol): 117.3
3. Isotonic specific volume (90.2K ): 323.2
4. Surface tension (dyne/cm): 57.5
5. Polarizability (10-24cm3): 15.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Extension�Molecular polar surface area 37.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 200
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Keep away from oxides.
Storage method
Store in an airtight container in a cool, dry place. Store away from oxidizing agents.
Synthesis method
1. Preparation method:
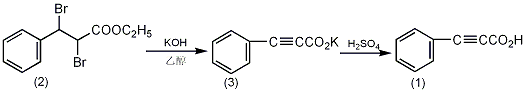 In a reaction bottle equipped with a stirrer and a reflux condenser , add 1.2L of 95% ethanol and 252g of potassium hydroxide (4.5mol), stir to dissolve. Cool to 40~50°C, add 336g (1.0mol) of α,β-dibromo-βphenylpropionic acid ethyl ester (2), and heat to reflux for 5 hours after the reaction becomes stable. Cool, filter out the precipitate (retained), adjust the filtrate to neutrality with concentrated hydrochloric acid, then filter out the precipitate (retained), and recover ethanol by distillation under normal pressure. Combine the residue with the precipitate (3) filtered above, dissolve it in 800 mL of water, and add crushed ice to make it about 1.8L. Use 20% sulfuric acid in an ice water bath to make it highly acidic ①, and a light brown solid will precipitate. Filter it with suction and wash with 2% dilute sulfuric acid. Dissolve the filtered solid in 1.5L (5%) sodium carbonate, decolorize with activated carbon, filter with suction, cool and add 200g of crushed ice. Adjust to strong acidity with 20% sulfuric acid, precipitate the solid, suction filter, wash with 2% sulfuric acid and water in sequence, and dry in a vacuum dryer to obtain 115g of phenylpropinoic acid (1), with a yield of 79%. Recrystallize with carbon tetrachloride to obtain 70g of pure product, mp 135~136℃. Note: ① When adding sulfuric acid, it should be done at low temperature as much as possible to prevent decarboxylation reaction. [1]
In a reaction bottle equipped with a stirrer and a reflux condenser , add 1.2L of 95% ethanol and 252g of potassium hydroxide (4.5mol), stir to dissolve. Cool to 40~50°C, add 336g (1.0mol) of α,β-dibromo-βphenylpropionic acid ethyl ester (2), and heat to reflux for 5 hours after the reaction becomes stable. Cool, filter out the precipitate (retained), adjust the filtrate to neutrality with concentrated hydrochloric acid, then filter out the precipitate (retained), and recover ethanol by distillation under normal pressure. Combine the residue with the precipitate (3) filtered above, dissolve it in 800 mL of water, and add crushed ice to make it about 1.8L. Use 20% sulfuric acid in an ice water bath to make it highly acidic ①, and a light brown solid will precipitate. Filter it with suction and wash with 2% dilute sulfuric acid. Dissolve the filtered solid in 1.5L (5%) sodium carbonate, decolorize with activated carbon, filter with suction, cool and add 200g of crushed ice. Adjust to strong acidity with 20% sulfuric acid, precipitate the solid, suction filter, wash with 2% sulfuric acid and water in sequence, and dry in a vacuum dryer to obtain 115g of phenylpropinoic acid (1), with a yield of 79%. Recrystallize with carbon tetrachloride to obtain 70g of pure product, mp 135~136℃. Note: ① When adding sulfuric acid, it should be done at low temperature as much as possible to prevent decarboxylation reaction. [1]
Purpose
None

 微信扫一扫打赏
微信扫一扫打赏

