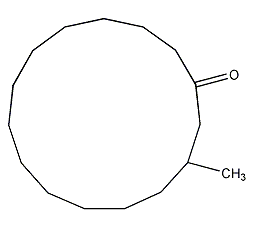
Structural formula
| Business number | 05L7 |
|---|---|
| Molecular formula | C16H30O |
| Molecular weight | 238.41 |
| label |
3-methylcyclopentadecanone, 3-methylcyclododecanone, 3-methylcyclopentadecanone, 3-methyl-1-cyclopentadecanone, dl-Muscone |
Numbering system
CAS number:541-91-3
MDL number:None
EINECS number:208-795-8
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Properties: colorless oily liquid
2. Refractive index at room temperature (n20): 1.480217
3. Relative steam density (g/cm3, air=1): 0.9221
4. Relative density (20℃, 4℃): 0.922117
5. Boiling point (ºC, normal pressure): 328
6. Boiling point (ºC, 66.70kPa): 130
7. Refractive index : 1.4802
8. Flash point (ºF): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition Combustion temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 55.1ºC): Undetermined
p>
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined Determined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
1. Skin/eye irritation data: Standard Draize test rabbit direct contact with skin: 500mg/24HREACTION SEVERITY: moderate;
2. Acute toxicity: rat oral LD50: >5gm/kg, No details other than lethal dose;
Mouse intraperitoneal LD50: 270mg/kg, Behavior – changes in sleep duration (including changes in righting reflex), convulsions or epilepsy, effects on the lungs, chest or breathing and other changes;
Mouse intravenous LD50: 152 mg/kg, no detailed description except lethal dose;
Rabbit transdermal LD50: >5gm/kg, except lethal dose No detailed description;
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 74.01
2. Molar body� (cm3/mol): 282.5
3. Isotonic specific volume (90.2K): 644.5
4. Surface tension (dyne/cm): 27.0
5. Polarizability (10-24cm3): 29.34
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 6.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 3
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 198
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Colorless oily liquid. The boiling point is 90°C, 130°C (66.7Pa), the relative density is 0.9221 (17/4°C), and the refractive index is 1.4802 (17°C).
Preparation method: There are many synthesis methods for muscone. The raw materials can be synthesized from cyclohexanone, butadiene, cyclopentadecanone, etc. The method using butadiene as raw material is one of the most studied and promising methods at present. Butadiene is extracted from butadiene to produce dodecacarbon-1,5,9-triene, which is then hydrogenated and oxidized into Cyclododecanone is then subjected to multi-step reactions to obtain muscone, with a yield of up to 40%. Muskone is the main fragrance component of natural musk, generally containing 1.2%-1.4% of muscone. The natural musk is steam distilled for several days, extracted and distilled with ether, and made into semicarbazone, then refined, decomposed, and finally refined by distillation.
Uses: Muscone is a representative species of macrocyclic musk. The basic chemical structure of macrocyclic musk is 13-19 acyclic and contains at least one functional group. According to the chemical structure, it can be divided into cyclic esters, Cyclic lactones, cyclic oxygen-containing ketones, cyclic ketone lactones, etc. Among the musk-type synthetic fragrances, the production of macrocyclic musk accounts for 5%. Musk ketone has a sweet aroma and can blend well with various spices. The aroma threshold is extremely low (0.001ppm-0.01ppm), the fragrance lasts long, and it is an extremely effective fixative. Pharmacologically, it has the effect of stimulating the central nervous system, respiratory center and heart, and promoting the secretion of various urea bodies in drought. It is an important drug for treating delirium. It can protect coronary arteries, increase coronary flow, promote blood circulation, reduce swelling and relieve pain. In addition, it can excite the uterus and enhance the contraction of uterine smooth muscle, so it should not be used by pregnant women. Musk is famous for its ability to open various orifices, open meridians, penetrate muscles and bones, internally treat stroke, qi, evil and infantile convulsions, and externally treat injuries caused by iron blows and sores. Adding musk to high-end calligraphy and painting not only makes the calligraphy and painting elegant and fragrant, but also has the special function of making the calligraphy and painting durable, water-resistant, and anti-corrosion.
Storage method
Stored in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. They should be stored separately from acids and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
There are many synthesis methods for muscone, and the raw materials can be selected from cyclohexanone, butadiene, cyclopentadecanone, etc. The method using butadiene as raw material is one of the most studied and promising methods at present. Butadiene is extracted from butadiene to produce dodecacarbon-1,5,9-triene, which is then hydrogenated and oxidized into Cyclododecanone is then subjected to multi-step reactions to obtain muscone, with a yield of up to 40%. Muskone is the main fragrance component of natural musk, generally containing 1.2%-1.4% of muscone. The natural musk is steam distilled for several days, extracted and distilled with ether, and made into semicarbazone, then refined, decomposed, and finally refined by distillation.
Uses: Muscone is a representative species of macrocyclic musk. The basic chemical structure of macrocyclic musk is 13-19 acyclic and contains at least one functional group. According to the chemical structure, it can be divided into cyclic esters, Cyclic lactones, cyclic oxygen-containing ketones, cyclic ketone lactones, etc. Among the musk-type synthetic fragrances, the production of macrocyclic musk accounts for 5%. Musk ketone has a sweet aroma and can blend well with various spices. The aroma threshold is extremely low (0.001ppm-0.01ppm), the fragrance lasts long, and it is an extremely effective fixative. Pharmacologically, it has the effect of stimulating the central nervous system, respiratory center and heart, and promoting the secretion of various urea bodies in drought. It is an important drug for treating delirium. It can protect coronary arteries, increase coronary flow, promote blood circulation, reduce swelling and relieve pain. In addition, it can excite the uterus and enhance the contraction of uterine smooth muscle, so it should not be used by pregnant women. Musk is famous for its ability to open various orifices, open meridians, penetrate muscles and bones, internally treat stroke, qi, evil and infantile convulsions, and externally treat injuries caused by iron blows and sores. Adding musk to high-end calligraphy and painting not only makes the calligraphy and painting elegant and fragrant, but also has the special function of making the calligraphy and painting durable, water-resistant, and anti-corrosion.
Purpose
Muscone is a representative species of macrocyclic musk. The basic chemical structure of macrocyclic musk is 13-19 acyclic and contains at least one functional group. According to the chemical structure, it can be divided into cyclic esters, cyclic lactones, and ring-containing musk. Oxyketones, cyclic ketone lactones, etc. Among the musk-type synthetic fragrances, the production of macrocyclic musk accounts for 5%. Musk ketone has a sweet aroma and can…��The various spices are well blended. The aroma threshold is extremely low (0.001ppm-0.01ppm), the fragrance lasts long, and it is an extremely effective fixative. Pharmacologically, it has the effect of stimulating the central nervous system, respiratory center and heart, and promoting the secretion of various urea bodies in drought. It is an important drug for treating delirium. It can protect coronary arteries, increase coronary flow, promote blood circulation, reduce swelling and relieve pain. In addition, it can excite the uterus and enhance the contraction of uterine smooth muscle, so it should not be used by pregnant women. Musk is famous for its ability to open various orifices, open meridians, penetrate muscles and bones, internally treat stroke, qi, evil and infantile convulsions, and externally treat injuries caused by iron blows and sores. Adding musk to high-end calligraphy and painting not only makes the calligraphy and painting elegant and fragrant, but also has the special function of making the calligraphy and painting durable, water-resistant, and anti-corrosion.

 微信扫一扫打赏
微信扫一扫打赏

