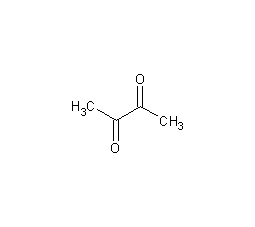
Structural formula
| Business number | 04SX |
|---|---|
| Molecular formula | C4H6O2 |
| Molecular weight | 86.09 |
| label |
diacetyl, diacetyl, diacetyl, Diacetyl, Butanedione, Biacetyl, Dimethyl diketone, food spices |
Numbering system
CAS number:431-03-8
MDL number:MFCD00008756
EINECS number:207-069-8
RTECS number:EK2625000
BRN number:605398
PubChem number:24846980
Physical property data
1. Properties: light yellow-green oily liquid. It has the smell of benzoquinone, and the extremely dilute aqueous solution has a special butter oil aroma. The vapor has a chlorine-like smell.
2. Relative density (g/mL, 18.5/4℃): 0.9808
3. Relative density (20℃, 4℃): 0.980818.5
4. Melting point (ºC): -1.2
5. Boiling point (ºC, normal pressure): 88~89
6. Refractive index at room temperature (n20D): 1.3951
7. Refractive index (18ºC): 1.3933
8. Flash point (ºC): 7
9. Specific rotation (º): Undetermined
10. Gas phase standard heat of combustion (enthalpy) (kJ·mol -1): -2104.4
11. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -327.1
12. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2065.7
13. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -365.8
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Explosion Lower limit (%, V/V): Undetermined
19. Solubility: Miscible with ethanol and ether, soluble in about 4 parts of water.
Toxicological data
1. Acute toxicity: Oral – Rat LD50: 1580 mg/kg; Oral – Mouse LD50: 250 mg/kg.
2. Irritation data: Skin – Rabbit 500 mg/24 hours Moderate.
3. Highly flammable and its vapor is harmful if inhaled. Irritating to eyes, respiratory system and skin.
Ecological data
��Substances may be harmful to the environment and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 20.63
2. Molar volume (cm3/mol): 88.8
3. Isotonic specific volume (90.2K ): 201.4
4. Surface tension (dyne/cm): 26.4
5. Polarizability (10-24cm3):8.18
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 71.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Natural products are found in laurel oil, celery seed oil, angelica root oil, raspberries, strawberries, cream, wine, etc. Because it is volatile, it only exists in the initial distillate of essential oils and distilled water. It has high vapor pressure and can evaporate quickly at room temperature. Miscible in ethanol, ether, most non-volatile oils and propylene glycol, soluble in glycerin and water, insoluble in mineral oil. Protective clothing should be worn when using.
2. Exist in flue-cured tobacco leaves, oriental tobacco leaves, and smoke.
3. Naturally found in the essential oils of Finnish pine, angelica and lavender, as well as Reunion geranium oil, Guava citronella oil and Laibai should be in the oil.
4. It is mostly generated when plant raw materials undergo water vapor.
Storage method
This product should be sealed and stored in a cool place. Storage temperature 4ºC
Synthesis method
1. In nature, diacetyl is widely found in essential oils of various plants, such as iris oil, angelica oil, laurel oil, etc. It is the main component of the fragrance of butter and other natural products. Industrial production of diacetyl is to treat methyl ethyl ketone with nitrous acid to generate butanone oxime, which is then decomposed with dilute sulfuric acid. Or use vinyl acetylene or methyl vinyl ketone to hydrate and then oxidize. In the laboratory, it can be prepared by oxidizing butanone with selenium dioxide, or by reacting diketoxime with sodium nitrite.
2. It can be obtained from high-content essential oils by the free method: add 1 part of essential oil to 2 parts of phosphoric acid to form a crystalline adduct C4H6O2·2H3PO4, and diacetyl will be released after adding water, such as If phosphoric acid is added in excess, the adduct will be liquid.
Obtained from glucose through special fermentation.
Synthesized using methyl ethyl ketone as starting material.
It is obtained by oxidizing butanone with sodium nitrite in the presence of hydrochloric acid, hydrolyzing it with sulfuric acid and then distilling it.
3. Tobacco: OR, 18; FC, 40.
4. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and dropping funnel (the lower end extends below the liquid level), add 2- Add 40g (0.56mol) of butanone (2), 52mL of concentrated hydrochloric acid, and dropwise add a solution of 40g (0.58mol) of sodium nitrite dissolved in 136mL of water at 50 to 60°C with vigorous stirring, and finish the addition in about 2 hours. Watch for nitric oxide escaping. After the addition is completed, transfer the reactants to a 5L reaction bottle, add a solution of 141g sodium nitrite dissolved in 240mL water, install air condensation, add dropwise a solution of 120mL concentrated sulfuric acid and 340mL water from the top of the condenser, and finish the addition in 10 minutes. . Leave the mixture for 2 days until the nitrite disappears (can be tested with potassium iodide-starch test paper). Add 200g of sodium sulfate and distill. Stop distillation when the distillate sample remains turbid after being mixed with 20% sodium hydroxide solution. The distilled liquid was dried with anhydrous sodium sulfate, re-distilled, and the fraction at 86-88°C was collected. The crude product thus obtained was dried over anhydrous sodium sulfate, distilled, and the fraction at 86-88°C was collected to obtain 16-18 g of 2,3-butanedione (1), with a yield of 33.3%-37.5%. [1]
Purpose
1. It is mainly used to prepare food flavors. It is the main spice of cream flavor. It can also be used in milk, cheese and other flavors. Such as berries, caramel, chocolate, coffee, cherries, vanilla beans, honey, cocoa, fruity aroma, wine aroma, tobacco aroma, rum, nuts, almonds, ginger, etc. It can also be used in trace amounts in fresh fruity fragrances for cosmetics or new flavors. Used as solvent and organic synthesis intermediate.
2. It can also be used as a hardener for gelatin. It is an important reagent for identifying nickel.

 微信扫一扫打赏
微信扫一扫打赏

