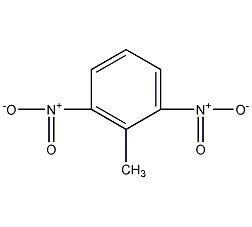
Structural formula
| Business number | 0692 |
|---|---|
| Molecular formula | C7H6N2O4 |
| Molecular weight | 182.14 |
| label |
2-Methyl-1,3-dinitrobenzene, CH3C6H3(NO2)2 |
Numbering system
CAS number:606-20-2
MDL number:MFCD00007158
EINECS number:210-106-0
RTECS number:XT1925000
BRN number:2052046
PubChem number:24869587
Physical property data
Physical property data:
1. Characteristics: Light yellow needle-like crystal.
2. Density (g/mL ,25/4℃):1.2833
3. Melting point (℃):66
4. Boiling Point (ºC):300
5. Solubility:Soluble in ethanol.
6. Toxicity:LD50177mg/kg(Rat orally)
Toxicological data
None
Ecological data
3. Ecological data:
1, other harmful effects: This substance may be harmful to the environment, Bodies of water should be given special attention.
Molecular structure data
5. Molecular property data: 1、 Molar refractive index:44.16 2、 Molar volume (m3/mol):129.3 3、 Isotonic specific volume (90.2K):355.8 4、 Surface tension (): 57.2 5, Polarizability (10-24cm3 ):17.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 91.6
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 198
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
Can evaporate with water vapor.
Storage method
2. Storage
This product should be kept sealed.
Synthesis method
3. Brief production method�
Obtained from the nitration of o-nitrotoluene with mixed acid. Production process and2,4-Same as dinitrotoluene.
Purpose
4. Purpose
Mostly used in industry2,4-Dinitrotoluene and2,6-Dinitrotoluene The mixture is reduced to 2,6/2,4-Diaminotoluene is then phosgenated to produce toluene Diisocyanate (TDI). It can also be used in other organic synthesis.

 微信扫一扫打赏
微信扫一扫打赏

