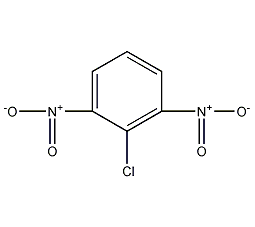
Structural formula
| Business number | 0693 |
|---|---|
| Molecular formula | C6H3ClN2O4 |
| Molecular weight | 202.55 |
| label |
2,6-dinitrochlorobenzene, 2,6-Dinitrochlorobenzene, Analytical reagents |
Numbering system
CAS number:606-21-3
MDL number:None
EINECS number:210-107-6
RTECS number:CZ0525500
BRN number:None
PubChem ID:None
Physical property data
1. Properties: Yellow needle-like crystals.
2. Density (g/mL, 16/4℃): 1.6867
3. Melting point (℃): 88
4. Boiling point (ºC): 315
p>
5. Solubility: soluble in ethanol and ether, insoluble in water.
Toxicological data
None
Ecological data
Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 44.23
2. Molar volume (cm3/mol): 125.0
3. Isotonic specific volume (90.2K): 354.1
4. Surface tension (dyne/cm): 64.2
5. Polarizability (10-24cm3): 17.53
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: None
3. Number of hydrogen bond acceptors: None
4. Number of rotatable chemical bonds: None
5. Number of tautomers: None
6. Topological molecular polar surface area None
7. Number of heavy atoms: None
8. Surface charge: None
9. Complexity: None
10. Number of isotope atoms: None
p>
11. Determine the number of atomic stereocenters: None
12. Uncertain number of atomic stereocenters: None
13. Determine the number of chemical bond stereocenters: None
14. Number of uncertain chemical bond stereocenters: None
15. Number of covalent bond units: None
Properties and stability
The product may not decompose under normal temperature and pressure.
Toxic. It has a significant irritating effect on the skin and mucous membranes, can cause severe dermatitis and blood poisoning, and damage the liver, kidneys and nervous system, leading to neuralgia, neuritis and neuromyositis. The maximum allowable concentration in the air is 1mg/m3. Operators should wear protective equipment, and clothes should be washed promptly after contamination.
Storage method
Seal the secret container and store it in a sealed main container in a cool, dry place.
Synthesis method
It is obtained by diazotization and substitution of 2,6-dinitroaniline. Add sodium nitrite to concentrated sulfuric acid, stir, and heat to 70°C. After all is dissolved, cool the solution to 25-30°C. Slowly add the solution prepared by 2,6-dinitroaniline and glacial acetic acid, and control the addition speed to keep the reaction temperature above 40°C. After adding, stir at 40℃half an hour. Then add pre-cooled concentrated hydrochloric acid solution of cuprous chloride, the reaction is exothermic and bubbles are generated. When the reaction becomes gentle, heat to 80°C and stop bubbling after 20 minutes. Add an equal volume of water, cool, filter, wash with water, dry, and recrystallize with acetic acid to obtain 2,6-dinitrochlorobenzene. The yield is about 70%.
Purpose
Dye intermediates. Used in the preparation of sulfur dyes. Also used as analytical reagents.

 微信扫一扫打赏
微信扫一扫打赏

