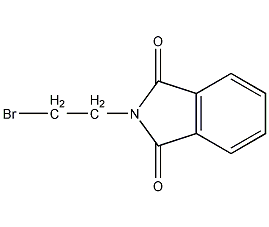
Structural formula
| Business number | 05US |
|---|---|
| Molecular formula | C10H8BrNO2 |
| Molecular weight | 254.08 |
| label |
N-β-Bromoethylphthalimide, β-Bromoethyl phthalimide, Phthaloyl ethyl imide bromide, N-(2-bromobenzyl)phthalimide, 1-Bromo-2-phthalimidoethane, Pharmaceutical R&D |
Numbering system
CAS number:574-98-1
MDL number:MFCD00005902
EINECS number:209-379-9
RTECS number:None
BRN number:148736
PubChem number:24891924
Physical property data
1. Physical property data
1. Properties: White needle-like crystals.
2. Melting point (℃): 82-83.5
3. Solubility: Easily soluble in ether and decomposed in hot water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 54.70
2. Molar volume (cm3/mol): 153.3
3. Isotonic specific volume (90.2K): 422.1
4. Surface tension (dyne/cm): 57.4
5. Polarizability (10-24cm3): 21.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 37.4
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 243
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
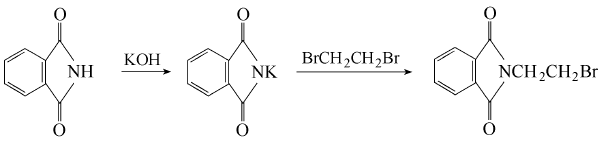
Phthalic anhydride is obtained by condensation and bromination: 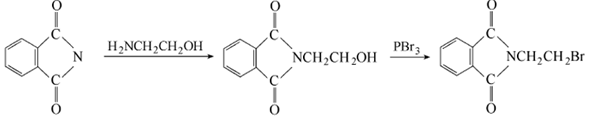 Put phthalic anhydride into the reaction pot Formic anhydride, slowly add ethanolamine, heat to 110°C to evaporate the water, and then vacuum dry for 2 hours. Add phosphorus tribromide dropwise at 110-120°C. After the addition is completed, keep stirring for 1.5 hours. Cool to 80°C, put the material into crushed ice to decompose excess phosphorus tribromide, leave it overnight to obtain crystals, centrifuge to dryness, recrystallize with ethanol, and dry at about 60°C to obtain the finished product. Add 18.5g phthalimide potassium salt and 94g 1,2-dibromoethane into the reactor, react at 90°C for 3 hours under nitrogen protection, and then cool , the reaction solution was washed with water until neutral, and dried over anhydrous magnesium sulfate. Excess 1,2-dibromoethane was recovered by distillation, and a yellow solid precipitated. The solid was recrystallized with acetone to obtain 22.1g of white needle-shaped solid, with a yield of 87.0%. Add 19.1gN-(2-hydroxyethyl)phthalimide and 100mL of chloroform to the reactor, heat to make the chloroform in a slight reflux state, here At room temperature, hydrogen bromide gas was introduced for 5 hours. After the reaction is completed, the reaction solution is washed with warm water until neutral, and the organic layer is dried with anhydrous sodium sulfate. Filter and evaporate the chloroform to obtain a yellow solid, which is recrystallized with acetone to obtain 22.3 g of white needle-shaped solid, with a yield of 87.8%.
Put phthalic anhydride into the reaction pot Formic anhydride, slowly add ethanolamine, heat to 110°C to evaporate the water, and then vacuum dry for 2 hours. Add phosphorus tribromide dropwise at 110-120°C. After the addition is completed, keep stirring for 1.5 hours. Cool to 80°C, put the material into crushed ice to decompose excess phosphorus tribromide, leave it overnight to obtain crystals, centrifuge to dryness, recrystallize with ethanol, and dry at about 60°C to obtain the finished product. Add 18.5g phthalimide potassium salt and 94g 1,2-dibromoethane into the reactor, react at 90°C for 3 hours under nitrogen protection, and then cool , the reaction solution was washed with water until neutral, and dried over anhydrous magnesium sulfate. Excess 1,2-dibromoethane was recovered by distillation, and a yellow solid precipitated. The solid was recrystallized with acetone to obtain 22.1g of white needle-shaped solid, with a yield of 87.0%. Add 19.1gN-(2-hydroxyethyl)phthalimide and 100mL of chloroform to the reactor, heat to make the chloroform in a slight reflux state, here At room temperature, hydrogen bromide gas was introduced for 5 hours. After the reaction is completed, the reaction solution is washed with warm water until neutral, and the organic layer is dried with anhydrous sodium sulfate. Filter and evaporate the chloroform to obtain a yellow solid, which is recrystallized with acetone to obtain 22.3 g of white needle-shaped solid, with a yield of 87.8%. 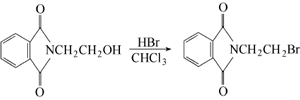
Purpose
Organic synthesis intermediates.

 微信扫一扫打赏
微信扫一扫打赏

