at present, the styrene production methods in the world mainly include ethylbenzene dehydrogenation method, propylene oxide-styrene ((po/sm)) co-production method and pyrolysis gasoline extraction method (c8 extraction method).
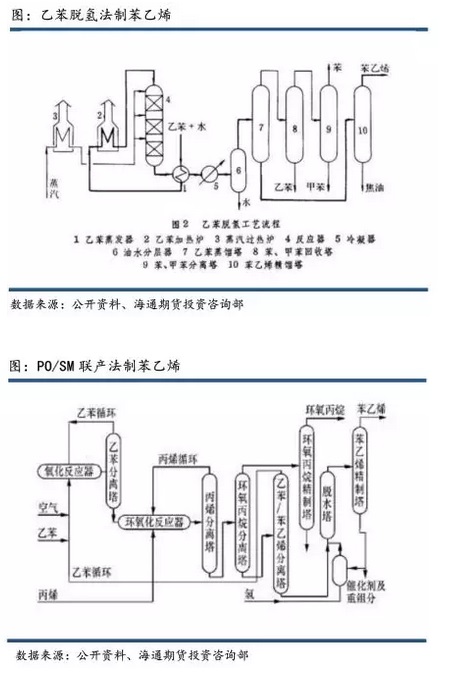
the mainstream process of global styrene is the ethylbenzene dehydrogenation method, which accounts for more than 80% of the global production share. that is, pure benzene and ethylene are used to produce ethylbenzene, and ethylbenzene is dehydrogenated to produce styrene. each ton of styrene usually requires 0.79 tons. benzene and 0.29 tons of ethylene. the ethylbenzene dehydrogenation process includes ethylbenzene catalytic dehydrogenation and ethylbenzene oxidative dehydrogenation. typical ethylbenzene catalytic dehydrogenation production processes include the fina/badger process, abb lummus/uop process and () process; the ethylbenzene oxidative dehydrogenation process is basically the same as the lummus/uop styrene process, but the reactor structure is different larger, the typical process is smart process.
the propylene oxide-styrene (po/sm) co-production method, also known as the co-oxidation method, was developed by halcon and perfected by american shell and other companies. this process currently accounts for about 12% of global production. the cogeneration method usually produces 2.6 tons of styrene and 1 ton of propylene oxide for every 3.2 tons of ethylbenzene and 0.8 tons of propylene.
the styrene extraction method from pyrolysis gasoline (c8 extraction method) refers to the pyrolysis gasoline produced from naphtha, diesel and liquefied petroleum gas as raw materials to steam cracking to ethylene equipment, which contains about 4-6% styrene. way to separate styrene. this method is low cost, but the product color is generally high, contains sulfur and fluctuates greatly. according to the exchange’s styrene delivery quality standards, styrene produced by this process cannot be used as a delivery product.

 微信扫一扫打赏
微信扫一扫打赏

