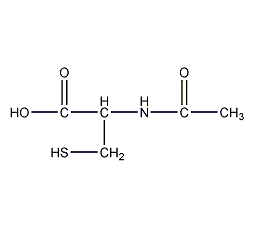
Structural formula
| Business number | 06HR |
|---|---|
| Molecular formula | C5H9NO3S |
| Molecular weight | 163.2 |
| label |
N-Acetyl-L-β-mercaptoalanine, N-acetylcysteine, Acetylcysteine, Phlegm is easy to clean; N-acetyl-3-mercaptoalanine, L-α-acetamido-β-mercaptopropionic acid, Acetylcysteine, LNAC, NAC, Airbron, Broncholysin, Inspir, Kespaire, Mucofilin, Mucomyst, L-α-Acetamido-β-mercaptopropionic acid, Phlegm dissolving medicine |
Numbering system
CAS number:616-91-1
MDL number:MFCD00004880
EINECS number:210-498-3
RTECS number:HA1660000
BRN number:1724426
PubChem number:24277970
Physical property data
1. Properties: White crystalline powder, with a garlic-like odor and a sour taste. Hygroscopic.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 109-110
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º, c=2, in water) : -35.1
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion Upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water or ethanol , insoluble in ether and chloroform.
Toxicological data
None yet
生�Academic data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 38.25
2. Molar volume (cm3/mol): 126.0
3. Isotonic specific volume (90.2K): 335.1
4. Surface tension (dyne/cm): 49.9
5. Polarizability (10-24cm3): 15.16
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 67.4
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 148
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It has a smell similar to garlic, tastes sour, and is hygroscopic.
Storage method
1. Sealed with argon and stored dry at 4ºC
Synthesis method
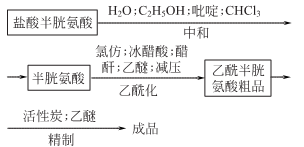
Synthesis method: 1. Dissolve L-cysteine hydrochloride (no crystal water) in 0.8 times distilled water, and heat appropriately at this time. Then filter it in a funnel with activated carbon. Add 3.6 times the volume of 95% medicinal ethanol to the filtrate, then add industrial pyridine (0.8 times the volume), cool it in an ice-water bath, then put it in the refrigerator overnight, filter it the next day, and use Wash with chloroform (industrial product), filter, press dry, quickly put into a vacuum dryer, and dry at room temperature for 5 hours to obtain L-cysteine. The yield is 86.5%.
It is obtained by acetylation of L-cysteine and acetic anhydride
Neutralization: Dissolve L-cysteine hydrochloride without crystal water in 0.8 times of distilled water. After dissolving in a water bath, pour it into a Buchner funnel pre-paved with activated carbon and suction filter. Take the filtrate and add ethanol (95%), then add pyridine, stir, put it in an ice water bath to cool, and then put it in the refrigerator overnight to precipitate. Crystallize, filter the crystals, wash with CHCl3, and press dry. Quickly put it into a vacuum dryer to dry to obtain L-cysteine.
Acetylation Put L-cysteine into the reaction tank, add glacial acetic acid, chloroform, and acetic anhydride under vigorous stirring. After the reaction tank interlayer is heated to about 35°C, stop heating, and the temperature in the tank gradually rises to 82-85°C. In order to control the temperature from being too high, start cooling at about 70°C, maintain it at about 50°C, stir for half an hour, and filter the filtrate. , and concentrate under reduced pressure. When it becomes thicker, add an appropriate amount of water to concentrate. Repeat this several times. When the concentrated solution is about 1.8 times of cysteine, cool it and add a few L-cysteine crystals to inoculate it. Place it in the refrigerator overnight to crystallize. Filter the crystals, press them to dryness, and wash them with ether to obtain Acetylcysteine crude product.
Dissolve the refined crude product in 0.5-0.6 times of distilled water, add 5% activated carbon for decolorization, filter the filtrate, and place it in the refrigerator overnight to precipitate crystals. Filter to collect the crystals, press to dryness, wash with ether, and dry under vacuum. Get Acetyl Cysteine Boutique.
2.
Purpose
1. Used as a phlegm-dissolving medicine. It is suitable for respiratory obstruction caused by a large amount of sticky phlegm. It can also be used to detoxify acetaminophen poisoning. Because this product has a special odor, taking it may cause nausea and vomiting. It has a stimulating effect on the respiratory tract and can cause bronchospasm. It is usually combined with bronchodilators such as isoproterenol and used with a sputum suction device to expel sputum. It is not suitable to come into contact with metals (such as Fe, Cu), rubber, oxidants, etc. It should not be used together with antibiotics such as penicillin, cephalosporin, tetracycline, etc. to avoid reducing its antibacterial effect. People with bronchial asthma should use with caution.
2. Used in biochemical research and medicinally as a phlegm dissolving agent and an antidote for acetaminophen poisoning.
3. Biochemical reagent, medicine. This product is used as an expectorant. It is said to be easy to clear up phlegm and easy to calm cough. It has a decomposing effect on thick phlegm. The mechanism of action is that the sulfhydryl group contained in the molecular structure of this product can break the disulfide bonds in the mucin polypeptide chain in mucus, decompose mucin, reduce the viscosity of sputum, and make it liquefied and easy to cough up. It is suitable for patients with acute and chronic respiratory diseases whose sputum is thick and difficult to cough up, as well as for critical symptoms caused by difficulty in breathing due to obstruction of large amounts of sticky sputum.
4. Protect mercaptool reagent.

 微信扫一扫打赏
微信扫一扫打赏

