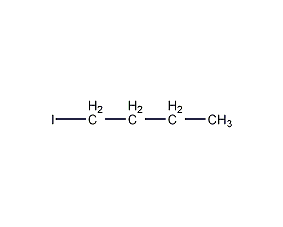
Structural formula
| Business number | 05LP |
|---|---|
| Molecular formula | C4H9I |
| Molecular weight | 184.02 |
| label |
Butane iodide, iodobutane, butyl iodide, n-butyl iodide, n-butane iodide, 1-iodobutane, Butyl iodide, n-Butyl iodide, n-Iodobutane, CH3CH2CH2CH2I, fungicides, analytical reagents, Halogenated hydrocarbon solvents, aliphatic compounds |
Numbering system
CAS number:542-69-8
MDL number:MFCD00001098
EINECS number:208-824-4
RTECS number:EK4400000
BRN number:1420755
PubChem ID:None
Physical property data
1. Properties: colorless liquid[1]
2. Melting point (℃): -130[2]
3. Boiling point (℃): 130~131[3]
4. Relative density (water=1): 1.62[4]
5. Relative vapor density (air=1): 5[5]
6. Critical pressure (MPa): 4.32[ 6]
7. Octanol/water partition coefficient: 3.060[7]
8. Flash point (℃): 33.33[8]
9. Ignition temperature (℃): 281[9]
10. Explosion limit (%) : No data yet
11. Lower explosion limit (%): 1.4[10]
12. Solubility: insoluble in water, soluble in ethanol, Ether, chloroform. [11]
Toxicological data
1. Acute toxicity[12]
LD50: 101mg/kg (mouse abdominal cavity); 620mg/kg (rat abdominal cavity)
LC50: 6100mg/m3 (rat inhalation, 4h)
2. Irritation No information available
p>
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 33.54
2. Molar volume (cm3/mol): 113.3
3. Isotonic specific volume (90.2K ): 265.2
4. Surface tension (dyne/cm): 29.9
5. Polarizability (10-24cm3): 13.30
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 13.1
10. Isotopes Number of atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine chemical bonds Number of stereocenters: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Discoloration when exposed to light or left for a long time. It is flammable and may cause combustion and explosion hazards when exposed to open flames, high heat, or oxidants. It decomposes when heated to release toxic iodide vapor. It is prone to nucleophilic substitution reactions; it can be reduced to butane by concentrated hydrogen iodide; it can generate octane through Wurtz reaction; it can generate butene by azeotrope with potassium hydroxide alcohol solution.
2. Stability[13] Stable
3. Incompatible substances[14] Strong oxidizing agent, strong alkali
4. Polymerization hazard[15] No polymerization
5. Decomposition products[16] Hydrogen iodide
Storage method
Storage Precautions[17] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Avoid light. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the reaction of n-butanol and iodine in the presence of phosphorus. Mix red phosphorus and n-butanol, heat to slightly reflux, and slowly add iodine. After the addition, continue to reflux for 4 hours and distill to obtain crude product. Wash with water, concentrated hydrochloric acid, water, 10% sodium carbonate solution and a small amount of sodium thiosulfate and water in sequence. Then dry with anhydrous calcium chloride, distill, and collect the 129-131°C fraction, which is 1-iodobutane. The yield is 90%.
Purpose
1. Used as analytical reagents, solvents, and organic synthesis. [18]

 微信扫一扫打赏
微信扫一扫打赏

