[overview]
dipotassium g lycyrrhizina te (dg) is a white or off-white powder, a triterpene saponin compound that is easily soluble in water, soluble in ethanol, and insoluble in oil. it contains hydrophilic properties groups and lipophilic groups, which can reduce the surface tension of aqueous solutions, have strong foaming power, and have the functions of emulsifying, dispersing, moisturizing and moisturizing hair, softening skin, anti-wrinkle, anti-sebum, preventing and treating pigmentation, anti-inflammatory and anti-itching, and cleaning and decontamination effective, widely used in medicine, daily chemicals, food and other industries. as a functional additive in cosmetics such as anti-allergy, sun protection, freckle removal, and skin repair, dipotassium glycyrrhizinate can effectively prevent sensitivity and inflammation when the skin is irritated, and has an anti-inflammatory and sedative effect on inflammation caused by sunlight. [1]
[physical and chemical properties]
molecular formula: c42h60k2o16
molecular weight: 899.13
dipotassium glycyrrhizinate is a white powder with a sweet taste, soluble in water, glycerol and propylene glycol, and slightly soluble in absolute ethanol and ether.
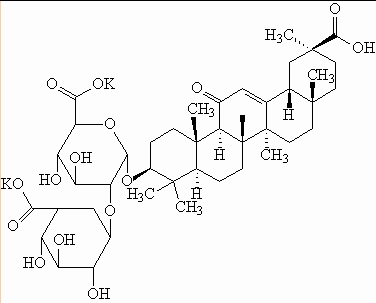
[preparation method]
dipotassium glycyrrhizinate is obtained by extracting licorice with water and then adding potassium hydroxide or potassium carbonate for complete neutralization.
[product identification][2-4]
common analysis methods for glycyrrhizic acid compounds include colorimetry, chromatography, atomic absorption, cracking/gc m/s coupled analysis technology, etc. the electrochemical polarographic analysis method has simple procedures and high sensitivity, and is often used for the determination of trace amounts of biomolecules, metal ions, and small drug molecules.
(1) take 0.2g of this product, add 5ml of water, and distill with 3ml of hydrochloric acid. add 2 to 3 drops of 2,4-dinitrophenylhydrazine ethanol test solution to the distillate to produce an orange-red precipitate.
(2) take the residue left under the ignition residue category of this product and check the identification reaction of potassium salt according to the law (appendix iii of part two of the chinese pharmacopoeia 2000 edition).
clarity: after dissolving 1.0g of this product in 20ml of water, the solution should be clear.
acidity: take 1.0g of this product, add 100ml of water to dissolve it, and measure it according to the law (chinese pharmacopoeia 2000 edition, part ii, appendix vi h). the ph value should be 5.0 to 6.0.
for chloride, take 0.5g of this product and check it according to the law (chinese pharmacopoeia 2000 edition, part ii, appendix viii a). compared with the control solution made of 7.0ml of standard sodium chloride solution, it must not be more concentrated (0.014%).
sulfate: take 0.5g of this product and check it according to the law (chinese pharmacopoeia 2000 edition, part ii, appendix viii b). compared with the control solution made of 1.5ml of standard potassium sulfate solution, it must not be more concentrated (0.029%).
heavy metals: take 1.0g of this product and check it according to the law (chinese pharmacopoeia 2000 edition, part ii, appendix viii h, second method). the heavy metal content should not exceed 20 parts per million.
take 1.0g of this product as arsenic salt and check it according to the law (chinese pharmacopoeia 2000 edition, part ii, appendix viii j, method 1), and it should comply with the regulations (0.0002%).
take 0.2g of this product for water content and check it according to the law (chinese pharmacopoeia 2000 edition, part ii, appendix viii m, method 1), and it should not exceed 8.0%.
take 1.0g of this product and check it according to the law (chinese pharmacopoeia 2000 edition, part ii, appendix viii n). the remaining residue should be 18.0% to 22.0% (calculated as anhydrous).
chromatographic conditions and system applicability: octadecylsilane bonded silica gel is used as the filler; acetonitrile-0.01mol/l phosphoric acid solution (38:62) is the mobile phase; the detection wavelength is 252nm. the number of theoretical plates calculated based on the monoammonium glycyrrhizinate peak should not be less than 2000, and the resolution of the monoammonium glycyrrhizinate peak and the peak of the internal standard substance should meet the requirements.
preparation of internal standard solution: take about 70 mg of n-butyl parahydroxybenzoate, weigh it accurately, place it in a 100 ml measuring bottle, dissolve it with dilute ethanol and dilute it to the mark, and shake well.
preparation of reference solution: take about 20 mg of monoammonium glycyrrhizinate reference substance, weigh it accurately, place it in a 100 ml measuring bottle, add dilute ethanol to dissolve it, and accurately add 5 ml of internal standard solution, dilute to the mark with dilute ethanol, and shake well .
preparation of test solution: take 20 mg of this product, weigh it accurately, place it in a 100 ml measuring bottle, add dilute ethanol to dissolve, and accurately add 5 ml of internal standard solution, then add dilute ethanol to dilute to the mark, and shake well.
[application and development prospects][5-6]
dipotassium glycyrrhizinate is a derivative of glycyrrhizic acid, which has anti-inflammatory, anti-allergic, anti-ulcer, and promotes epithelial cell tissue regeneration. the anti-inflammatory effect of 0.5% dipotassium glycyrrhizinate solution is equivalent to that of 0.1% dexamethasone, but there are no serious adverse reactions.
glycyrrhizic acid (dipotassium salt) and glycyrrhetinic acid have good preventive and therapeutic effects on oral diseases at different stages without any adverse reactions. there are also reports that dental pulp root canal sealants contain formaldehyde (paraformaldehyde, trimerformaldehyde), causing some patients to suffer from allergic shock after dental pulp root canal surgery. after adding dipotassium glycyrrhizinate, the allergic reaction symptoms are reduced or disappeared. . the recommended amount of dipotassium glycyrrhizinate in toothpaste is 0.1% to 0.5%.
[reference materials]
[1] liu xintong, li peinuan, wang yan, gao hongwei, wang qingxiang. polarographic determination of dipotassium glycyrrhizinate and its application [j]. chinese journal of health inspection, 2005(12):1435-1436+1459 .
[2]deng liyu, yao dehai, li tiezhu, et al. study on the determination of glycyrrhizic acid content in licorice by thin layer colorimetry [j]. chemical engineer, 2001, 1(1):35 – 36.
[3] shan yurong. determination of glycyrrhizic acid content in fengshi gutong tablets by high performance liquid chromatography [j]. chinese modern applied pharmacy, 2004, 21(1):1-47.
[4] lang huiyun, xie zhihai, zhao yi. application of atomic absorption method in organic analysis (v)——gananalysis method of glycyrrhizic acid in grass and its products [j]. chemical research and application, 1995, 7(1):72 – 75.
[5] yang fengkun et al. determination of glycyrrhizic acid content in compound licorice zinc oral composite film by reversed-phase high performance liquid chromatography [j]. tianjin medicine. 2009, 37(3): 221-222.
[6] wan xiuyu, zhu qideng. development of dipotassium glycyrrhizinate eye drops[j]. food and medicine. 2008, 10(1): 18-20.

 微信扫一扫打赏
微信扫一扫打赏

